CBSE Class 10 Science Answer Key 2023
Class 10 Science Answer Key 2023: CBSE has conducted the Class 10 Science Board Exam for 04th March 2023 (today) and the students, as well as their parents, must be eager to know the level of performance of their kids after the exam is over. The students can follow the Class 10 Science Answer Key 2023 which has been discussed in detail here by our faculty on 04th March 2023 itself. We are providing authentic and explained answers to each question asked in the Class 10 Science Examination 2023. Class 10 Science Exam Analysis for discussing Science Question Paper has been started by our faculty, join them and to know correct answers.
Science Class 10 Answer Key 2023
If you had your Class 10 Science Exam on 04th March 2023, then you must stay tuned with us so that you do not miss out on the updation of the Science Class 10 Answer Key 2023 that is been discussed by our faculty. The students can cross-check their responses to all the questions they attempted in the exam to the answers that will be provided here. After this, the students can begin their studies for the next exams.
| CBSE Class 10 Science Answer Key 2023 | |
| Exam Conducting Body | Central Board of Secondary Education |
| Exam Name | CBSE Class 10 Science |
| Category | Answer Key |
| Science Class 10 Exam Date | 04th March 2023 |
| Science Class 10 Answer Key (Unofficial) | 04th March 2023 (Today) |
| Official Website | https://www.cbse.nic.in/ |
Class 10 Science Answer Key 2023
The overall difficulty level of today’s Science Exam Paper was Easy to Moderate. As per the students, the overall difficulty level of each section has been tabulated below-
| Class 10 Science Question Paper 2023 Difficulty Level | |
| Sections | Difficulty Level |
| Section – A | Easy |
| Section – B | Easy to Moderate |
| Section – C | Easy to Moderate |
| Section – D | Easy to Moderate |
| Section – E | Easy to Moderate |
CBSE Class 10 Science Answer Key and Question Paper Solution
The students can refer to the CBSE Class 10 Science Answer Key 2023 which is been discussed by our expert faculty after the completion of the Science Board Exam on 04th March 2023 today. To get the accurate and detailed Science Class 10 Answer Key 2023 and Question paper solution, connect with our faculty and know all questions with their correct answers here.
Science Class 10 Questions & Solutions 2023 (31/1/2)
Check the correct answers to the questions asked in today’s 04th March 2023 Science Board Exam
Q1. Consider the structures of the three cyclic carbon compounds A, B, and C given below and select the correct option from the following-
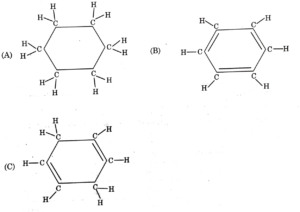
a) A and C are isomers of hexame and B is benzene
b) A is an isomer of hexame, B is benzene and C is an isomer of hexene.
c) A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons
d) A is cyclohexane and B and C are the isomers of benenze.
Correct Answer- c)
Q2. Select washing soda from the following-
a) NaHCO3
b) Na2CO3.5H2O
c) Na2CO3.10H2O
d) NaOH
Correct Answer- c)
Q3. Copper is used for making cooking utensils. Which of the following physical properties of copper is NOT responsible for the same?
a) Malleability
b) High Melting Point
c) Thermal Conductivity
d) High reactivity
Correct Answer- d)
Q4. The table below has information regarding pH and the nature (acidic/basic) of four different solutions. Which one of the options in the table is correct?
| Option | Solution | Colour of pH paper | Approximate pH value | Nature of Solution |
| a) | Lemon Juice | Orange | 3 | Basic |
| b) | Milk of Magnesia | Blue | 10 | Basic |
| c) | Gastric Juice | Red | 6 | Acidic |
| d) | Pure Water | Yellow | 7 | Neutral |
Correct Answer- b)
Q5. MnO2 + x HCl —— MnCl2 + y H2O + z Cl2
In order to balance the above chemical equation, the value of x, y, and z respectively are-
a) 6, 2, 2
b) 4, 1, 2
c) 4, 2, 1
d) 2, 2, 1
Correct Answer- c)
Q6. The emission of brown fumes in the given experimental set-up is due to-
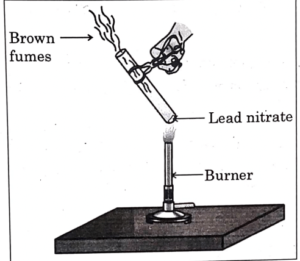
a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
b) thermal decomposition of lead nitrate which produces brown fumes of lead oxide.
c) oxidation of lead nitrate forming lead oxide and nitrogen dioxide.
d) oxidation of lead nitrate forming lead oxide and oxygen.
Correct Answer- a)
Q8. Select endothermic reaction from the following :
a) Decomposition of vegetable matter into compost.
b) Decomposition of calcium· carbonate to form quick lime and carbon dioxide.
c) Burning of a c~ndle.
d) Process of respiration.
Correct Answer- b)
CBSE Class 10 Science Answer Key 2023 (Code- 31/6/2) Section A
| CBSE Class 10 Science Answer Key 2023 (Code- 31/6/2) Section A | |
| 1- (b) | 11- (b) |
| 2- (c) | 12- (b) |
| 3- (b) | 13- (b) |
| 4- (a) | 14- (c) |
| 5- (b) | 15- (d) |
| 6- (c) | 16- (b) |
| 7- (a) | 17- |
| 8- (d) | 18- |
| 9- (c) | 19- |
| 10- (d) | 20 |
Section A
Select and write one most appropriate option out of the four options.
Q1. Metal oxides generally react with acids but few oxides of metal also react with bases. Such metallic oxides are-
I. MgO
II. ZnO
III. Al2O3
IV. CaO
a) I and II
b) II and III
c) III and IV
d) I and IV
Correct Answer- b) II and III
Q2. Few drops of aqueous solution of ammonium chloride are put on a universal indicator paper. The paper turns pink.
Study the following table and choose the correct option-
| Nature | Ammonium Chloride is a salt of | Range of pH |
| a) Acidic | weak acid and strong base | less than 7 |
| b) Basic | weak acid and weak base | more than 7 |
| c) Acidic | strong acid and weak base | less than 7 |
| d) Basic | strong acid and strong base | 7 |
Correct Answer- c)
Q3. Select the appropriate state symbols of the products given as X and Y in the following chemical equation by choosing the correct option from the table given below-
Zn(S) + H2 SO4 —— ZnSO 4 + H2
| (X) | (Y) | |
| a) | (s) | (l) |
| b) | (aq) | (g) |
| c) | (aq) | (s) |
| d) | (g) | (aq) |
Correct Answer- b)
Q4. Two salts “X” and “Y” are dissolved in water separately. When phenolphthalein is added to these two solutions, solution “X” turns pink and solution “Y” does not show any change in colour, therefore “X” and “Y” are-
Correct Answer- a)
Q5. In the given diagram of closed stomata. (1), (2), (3), and (4) respectively are-
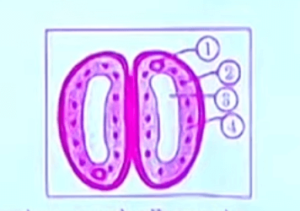
a) nucleus, chloroplast, guard cell, vacuole
b) nucleus, chloroplast, vacuole, guard cell
c) chloroplast, nucleus, vacuole, guard cell
d) vacuole, guard cell, nucleus, chloroplast
Correct Answer- b)
Q6. Walking in a straight line and riding a bicycle are activities which are possible due to a part of the brain. Choose the correct location and name of this part from the given table.
| Part of the Brain | Name | |
| a) | Fore Brain | Cerebrum |
| b) | Mid Brain | Hypothalamus |
| c) | Hind Brain | Cerebellum |
| d) | Hind Brain | Medulla |
Correct Answer- c)
Q7. A student wants to obtain an erect image of an object using a concave mirror of 10 cm focal length. What will be the distance of the object from mirror?
a) Less than 10 cm
b) 10 cm
c) between 10 cm and 20 cm
d) more than 20 cm
Correct Answer- a)
Q8. Bronze is an alloy of
a) Copper and Zinc
b) Aluminium and Tin
c) Copper, Tin, and Zinc
d) Copper and Tin
Correct Answer- d)
Q9. In an experiment with pen plants, a pure tall plant (TT) is crossed with pure short plant (tt). The ratio of pure tall plant to pure short plant in F2 generation will be
a) 1:3
b) 3:1
c) 1:1
d) 2:1
Correct Answer- c)
Q10. Study the given figure of food web and identify the primary consumer in the food web: 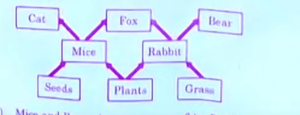
a) Mice and Bear
b) Rabbit and Cat
c) Rabbit and Fox
d) Mice and Rabbit
Correct Answer- d)
CBSE Class 10 Science Answer Key 2023 Set – 3
Q1. When aqueous solutions of potassium iodide and lead nitrate are mixed, an insoluble substance separates out. The chemical equation for the reaction involved is:
(a) KI+PbNO3 –> PbI + KNO3
(b) 2KI+Pb(NO3)2 –> PbI2 + 2KNO3
(c) KI+PbNO3)2 –> PbI + KNO3
(d) KI+PbNO3 –> PbI2 + KNO3
Correct Answer: (b)
Q2. When Sodium bicarbonate reacts with dilute hydrochloric acid, the gas evolved is:
(a) Hydrogen; it gives pop sound with burning match stick.
(b) Hydrogen; it turns lime water milky.
(c) Carbon dioxide; it turns lime water milky.
(d) Carbon dioxide; it blows off a burning match stick with a pop sound.
Correct Answer: (c)
Q3. Acid present in tomato is:
(a) Methanoic acid
(b) Acetic Acid
(c) Lactic acid
(d) Oxalic acid
Correct Answer: (d)
Q4. A metal ribbon ‘X’ burns in oxygen with a dazzling white flame forming a white ash ‘Y’. The correct description of X, Y and the type of reaction is:
(a) X=Ca;Y=CaO; Type of Reaction = Decomposition
(b) X=Mg;Y=MgO; Type of Reaction = Combination
(c) X=Al;Y=Al2O; Type of Reaction = Thermal Decomposition
(d) X=Zn;Y=ZnO; Type of Reaction = Endothermic
Correct Answer: (b)
Q5. The name of the salt used to remove permanent hardness of water is:
(a) Sodium hydrogen carbonate (NaHCO3)
(b) Sodium chloride (NaCl)
(c) Sodium carbonate decahydrate (Na2CO3.10H20)
(d) Calcium sulphate hemihydrate (CaSO4.1/2H20)
Correct Answer: (c)
Q6. The electron dot structure of chlorine molecule is:

Correct Answer: (c)
Q7. Sodium hydroxide is termed an alkali while Ferric hydroxide is not because:
(a) Sodium hydroxide is a strong base, while Ferric hydroxide was weak base.
(b) Sodium hydroxide is a base, which is soluble in water while Ferric hydroxide is also a base but it is not soluble in water.
(c) Sodium hydroxide is a strong base while Ferric hydroxide is strong acid
(d) Sodium hydroxide and Ferric hydroxide both are strong base but the solubility of Sodium hydroxide in water is comparatively higher than that of Ferric hydroxid.
Answer: b)
Q8. Opening and closing of stomata is due to:
(a) High pressure of gases inside the cells.
(b) Movement of water in and out of the guard cells
(c) Stimulus of light in the guard cells
(d) Diffusion of CO2 in and out of the guard cells
Correct Answer: b)
Q9. Water in the root enters due to:
(a) the function of the root to absorb water.
(b) difference in the concentration of ions between the root and the soil.
(c) excess water present in the soil
(d) diffusion of water in the roots.
Correct Answer: b)
Q10. Which one of the given statements is incorrect:
(a) DNA has the complete information for a particular characteristic
(b) DNA is the molecule responsible for the inheritance of characters from parents to offsprings
(c) Change in information will produce a different protein.
(d) Characteristics will remain the same even if protein changes
Correct Answer: d)
Q11. Sensory nerve of a reflex arc carries information from the receptor cells to the:
(a) spinal cord
(b) brain
(c) muscles of the effector organ
(d) bones of the receptor organ
Correct Answer: a)
Q12. A cross between pea plant with white flowers (vv) and pea plant with violet flowers (VV) resulted in F2 progeny in which ratio of violet (VV) and white (vv) flowers will be:
(a) 1:1
(b) 2:1
(c) 3:1
(d) 1:3
Correct Answer: c)
CBSE Class 10 Science Question Paper 2023 Scheme
The CBSE Class 10 Science Board Exam will be of descriptive nature for which the time allotted is 3 hours for 39 questions. The paper will be divided into 5 sections-
1. Section A consists of 20 objective-type questions carrying 1 mark each.
2. Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should in the range of 30 to 50 words.
3. Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should in the range of 50 to 80 words
4. Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
5. Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
Science Class 10 Question Paper 2023- (Code-31/1/2)
Science Class 10 Question Paper 2023- (Code-31/5/1)
Science Class 10 Question Paper 2023- (Code-31/6/1)
Class 10 Science Answer Key 2023- FAQs
Q1. What is the pattern for Class 10 Science Exam 2023?
Ans. Class 10 Science question paper consists of 39 questions divided into 5 sections.
Q2. Will I get CBSE Class 10 Science Answer Key 2023 after exam is over?
Ans. Yes, we are providing the Class 10 Science Answer Key 2023 in this article after the exam is over.
Q3. What was the level of today's Science Class 10 Question Paper?
Ans. As per students' review, today's Science Class 10 Question Paper was of Easy level.




 Rajasthan PTET Online Form 2026, Registr...
Rajasthan PTET Online Form 2026, Registr...
 UPSC CAPF Notification 2026 Out for 349 ...
UPSC CAPF Notification 2026 Out for 349 ...
 Rajasthan PTET Previous Year Question Pa...
Rajasthan PTET Previous Year Question Pa...







