Glucose is a crucial molecule in biology and has several important properties and functions. Glucose is a simple sugar and a vital source of energy for living organisms, is found in various forms throughout the natural world. It is throughout the natural world. It is primarily present in the bloodstream of animals, where it circulates to provide energy to cells and tissues. Additionally, glucose is a fundamental component of many carbohydrate-rich foods, such as fruits, vegetables, grains, and honey.
Plants synthesize glucose through photosynthesis, converting sunlight into energy and storing it as glucose in the form of starch. This stored energy source serves as the foundation for the food chain, as herbivores consume plants containing glucose, and carnivores, in turn, feed on herbivores.
What is Glucose?
Glucose is a type of sugar and a fundamental source of energy for living organisms, including humans. The Chemical formula for glucose is C6H12O6. It is a simple carbohydrate that our bodies use to produce energy through processes like cellular respiration. Glucose can be found in various foods, and it’s also produced in the body, primarily in the liver, where it’s stored as glycogen for future energy needs. Monitoring blood glucose levels is essential for managing conditions like diabetes, as it helps regulate the body’s energy supply.
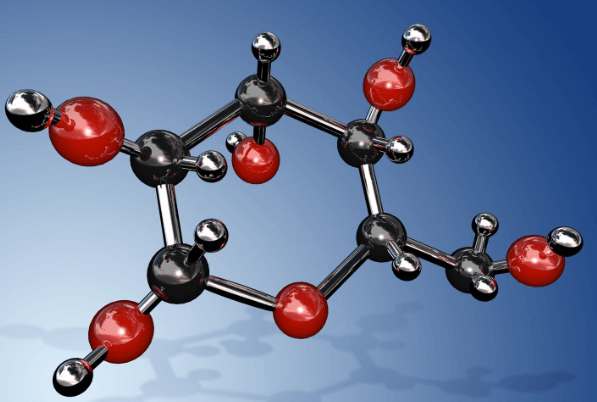
Structure of Glucose
Glucose is a six-carbon ring (hexose), with five hydroxyl (-OH) groups and one carbonyl (-C=O) group. The molecular structural formula is usually depicted in a linear or ring form, but in reality, glucose predominantly exists in a ring structure known as a six-membered pyranose ring. It can exist in two isomeric forms known as alpha and beta glucose, depending on the orientation of the -OH group at the first carbon atom in the ring.
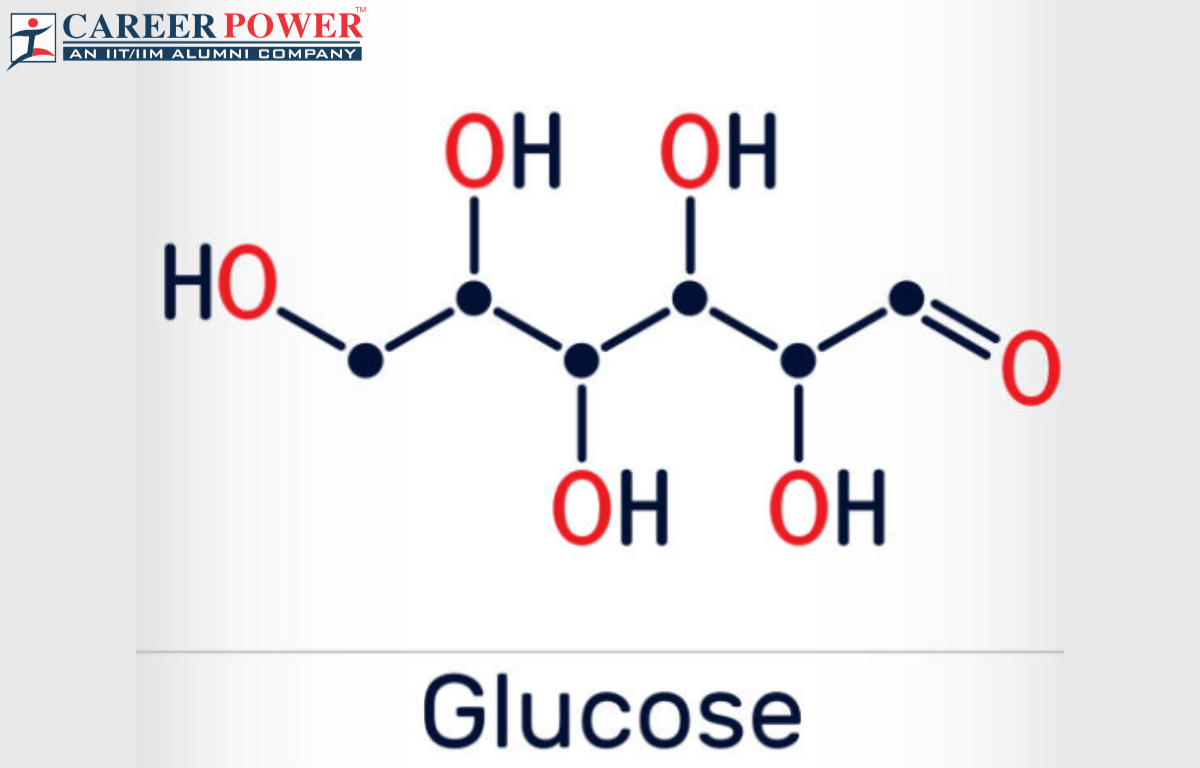
Linear Formula:
In a linear formula, glucose is often represented as a chain of six carbon atoms, each carrying a hydroxyl group (-OH), and a carboxyl group (C=O) on the first carbon atom. The linear formula is:
H—(C=O)—(CHOH)5—H
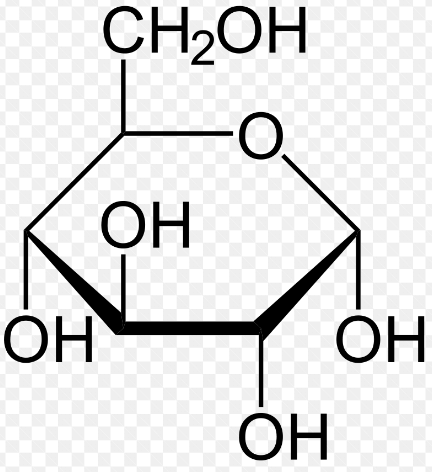
Ring Formula (Haworth Projection):
In reality, glucose existed mainly in a ring form. The most common ring structure for glucose is the six-membered ring, specifically the pyranose form. It can be represented in the Haworth projection. The ring structure is often referred to as a “hexose” because it has six carbon atoms.
Composition of Glucose
Glucose is a simple sugar with the chemical formula C6H12O6. It consists of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms arranged in a specific molecular structure. This structure is a hexagonal ring, with each carbon atom bonded to a hydrogen atom and either another carbon atom or an oxygen atom. Glucose is the fundamental molecule used by organisms for energy production and is a key component of carbohydrates.
Glucose is an example of an Aldohexose, which means it is a six-carbon sugar with an aldehyde functional group (-CHO) at one end of the molecule. There are different structural isomers of hexoses, but glucose has specific structural features. It forms a six-membered ring structure called a Pyranose ring, typically in its most stable form, which is alpha-D-glucose or beta-D-glucose.
Properties of Glucose
Glucose has several important properties that highlight the central role that plays in metabolism and energy production in living organisms.
- Solubility: Glucose is highly soluble in water, which makes it easily transported in the bloodstream.
- Sweetness: Glucose is moderately sweetness level lower than that of the table sugar (sucrose).
- Chemical Structure: It is a monosaccharide, a simple sugar, with a molecular formula C6H12O6. Its structural formula is a hexose, meaning it has six carbon atoms.
- Energy Source: Glucose is a primary source of energy for the human body and many other organisms. It is metabolized in the cell through processes like glycolysis and citric acid to produce ATP (adenosine triphosphate), which is used for energy.
- Blood Sugar Regulation: Maintaining appropriate blood glucose levels is crucial for overall health. The body’s hormonal systems, including insulin and glucagon, help regulate glucose levels to keep them within a narrow range.
- Storage as Glycogen: Excess glucose in the body is stored in the liver and muscles as glycogen, which can be converted back to glucose when energy is needed.
- Nutritional Importance: Glucose is a vital nutrient, and it provides calories or energy when consumed in foods and beverages. It is commonly found in carbohydrates, including starchy foods like bread, rice, and potatoes, as well as in fruits and some vegetables.
- Medical Significance: Monitoring blood glucose levels is essential for an individual with conditions like diabetes to manage their health effectively.
Preparation of Glucose
Glucose can be prepared through various methods, but one common method is the enzymatic hydrolysis of starch, typically derived from corn or potatoes. It’s important to note that this is a simplified overview and the actual industrial production of glucose can involve more complex processes and quality control measures. Additionally, glucose can also be obtained through other methods, such as the enzymatic hydrolysis of cellulose or from other carbohydrates found in nature. Here we have mentioned a simplified chemical equation for this reaction:
(C6H10O5)n + nH2O → nC6H12O6
In this equation:
- (C6H10O5)n represents starch, which is a polymer of glucose.
- n is the number of glucose units in the starch molecule.
- nH2O represents the water molecules needed for the hydrolysis reaction.
- Starch Extraction: Starch is extracted from a suitable source, such as corn or potatoes. This starch-rich material is usually ground into a create a starch slurry.
- Enzymatic Hydrolysis: Enzymes, such as amylase, are added to the starch slurry. Amylase enzymes break down the starch into similar sugars, including glucose, through the process of hydrolysis. This enzymatic reaction typically occurs at elevated temperatures to speed up the process.
- Hydrolysis Product Separation: After the enzymatic hydrolysis, the mixture is usually heated to stop the enzymatic activity. The resulting mixture contains various sugars, including glucose.
- Purification: The mixture is then purified to isolate glucose from other sugars and impurities. This may involve processes such as filtration, decolorization, and ion exchange chromatography.
- Crystallization: Glucose can be further purified and crystallization from the solution the solution to obtain solid glucose crystals.
- Drying: The glucose crystals are dried to remove any remaining moisture, resulting in the final product, which is often sold as glucose power or glucose syrup.
Uses of Glucose
As we know glucose is a very important monosaccharide that helps in various bodily functions.
- Energy Source: Glucose is a primary source of energy for the body’s cells. It is metabolized in cells through processes like glycolysis and the citric acid cycle to produce ATP, the cell’s energy currency.
- Medical Applications: Glucose is used intravenously in medical settings to treat patients with low blood sugar (hypoglycemia) or to provide energy support to those unable to eat.
- Food and Beverage Industry: Glucose is used as a sweetener, a thickening agent, and in various food products to enhance flavor, texture, and shelf life.
- Sports and Energy Drinks: Glucose is added to sports drinks and energy for athletes during physical activities.
- Laboratory Testing: Glucose is used in diagnostic tests like the oral glucose tolerance test (OGTT) to assess how the body processes sugar and to diagnose conditions such as diabetes.


 50 Vegetables Name for Kids in English a...
50 Vegetables Name for Kids in English a...
 Food Chain: Definition, Types, Examples,...
Food Chain: Definition, Types, Examples,...
 Human Respiratory System: Definition, Di...
Human Respiratory System: Definition, Di...













