What is Chemical Formula?
The chemical formula of a compound is a representation of a chemical composition that provides information about the types and numbers of atoms present in the compound using elemental symbols and subscripts. It shows the types and numbers of atoms that make up the compounds. The chemical formula of a compound provides essential information about its composition and structure.
Elemental symbols are abbreviations for the chemical elements present in the compound. Subscript numbers indicate the number of atoms of each element in a molecule of the compound. For example, H2O is the chemical formula for water, which indicates that it consists of two hydrogen (H) atoms and one oxygen (O) atom bonded together. The chemical formulas are essential for describing and communicating the composition of substances in chemistry. In this article, we are covering all chemical formulas used in chemistry for Class 9, 10, 11 and 12 standards along with the types types of chemical formulas.
Types of Chemical Formulas
Chemical formulas represent the composition of chemical compounds. Here we have mentioned some of the common types of chemical formulas used in chemistry to describe and represent different types of compounds and molecules.
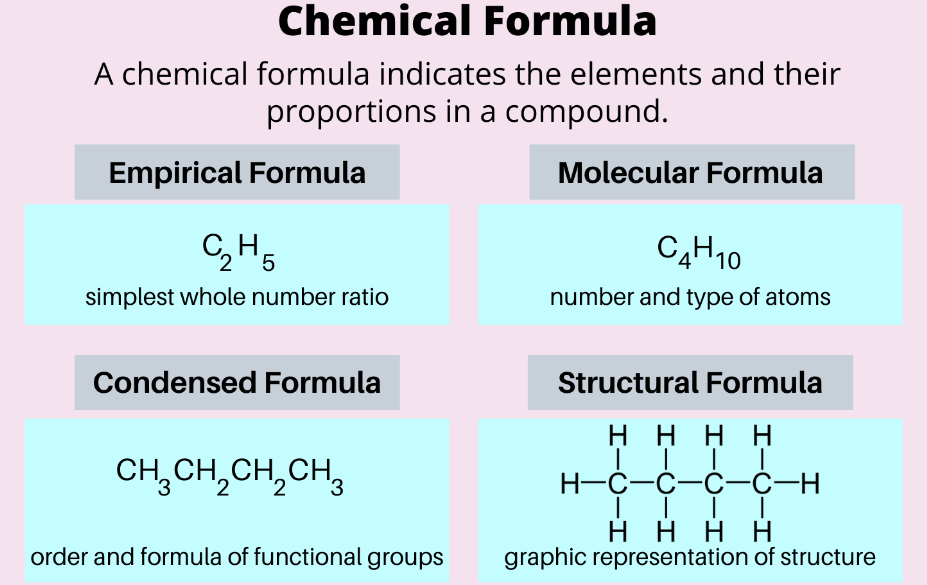
| Different Types of Chemical Formulas | ||
| Types of Formula | Description | Examples |
| Empirical Formula | The empirical formula is the simplest whole-number ratio of elements in a compound. | H2O2 (Hydrogen Peroxide). |
| Molecular Formula | The molecular formula is the actual number of atoms of each element in a molecule. | H2O2 (Hydrogen Peroxide). |
| Structural Formula | Uses lines to represent bonds and atom arrangement. | H2O (Water) |
| Condensed Structural Formula | Simplified structural formula with omitted bond lines. | CH3CH2OH (Ethanol) |
Empirical Formula
The empirical formula informs about the simplest whole-number or the most reduced ratio of elements in a given compound. The empirical formula doesn’t provide the exact number ratio of atoms but shows the relative proportions. For example, the empirical formula for hydrogen peroxide is H2O2.
Molecular Formula
The molecular formula shows the actual number of atoms of each element in a molecule. The molecular formula gives the exact composition of a compound. For example, the molecular formula for hydrogen peroxide is also H2O2.
Structural Formula
The structural formula uses lines to represent bonds between atoms and shows how atoms and shows how atoms are arranged in the molecule. It provides a detailed visual representation of a compound’s structure.
Condensed Structural Formula
The condensed formula is a variation of the structural formula that simplifies it further. It omits some bond lines and shows atoms grouped together. Using ethanol as an example, its condensed structural formula is CH3CH2OH.
Chemical Formula List (Table)
Here we have mentioned all the chemical compounds with their chemical formulas.
| Name of the Chemical Compound | Formula |
| Acetic acid formula | CH3COOH |
| Aluminum hydroxide formula | Al(OH)3 |
| Acetate formula | CH3COO- |
| Acetone formula | C3H6O |
| Aluminium acetate formula | C6H9AlO6 |
| Aluminium bromide formula | AlBr3 |
| Aluminium carbonate formula | Al2(CO3)3 |
| Aluminium chloride formula | AlCl3 |
| Aluminium fluoride formula | AlF3 |
| Aluminum formula | Al |
| Aluminium iodide formula | AlI3 |
| Aluminium oxide formula | Al2O3 |
| Aluminium phosphate formula | AlPO4 |
| Amino acid formula | H2NCHRCOOH |
| Ammonia formula | NH3 |
| Ammonium dichromate formula | Cr2H8N2O7 |
| Ammonium acetate formula | C2H3O2NH4 |
| Ammonium bicarbonate formula | NH4HCO3 |
| Ammonium bromide formula | NH4Br |
| Ammonium carbonate formula | (NH4)2CO3 |
| Ammonium chloride formula | NH4Cl |
| Ammonium hydroxide formula | NH4OH |
| Ammonium iodide formula | NH4I |
| Ammonium nitrate formula | NH4NO3 |
| Aluminium sulfide formula | Al2S3 |
| Ammonium nitrite formula | NH4NO2 |
| Ammonium oxide formula | (NH4)2O |
| Ammonium phosphate formula | (NH4)3PO4 |
| Ammonium sulfate formula | (NH4)2SO4 |
| Ammonium sulfide formula | (NH4)2S |
| Argon gas formula | Ar |
| Ascorbic acid formula | C6H8O6 |
| Barium acetate formula | Ba(C2H3O2)2 |
| Barium bromide formula | BaBr2 |
| Barium chloride formula | BaCl2 |
| Barium fluoride formula | BaF2 |
| Barium hydroxide formula | Ba(OH)2 |
| Barium iodide formula | BaI2 |
| Barium nitrate formula | Ba(NO3)2 |
| Barium oxide formula | BaO |
| Barium phosphate formula | Ba3O8P2 |
| Barium sulfate formula | BaSO4 |
| Benzene formula | C6H6 |
| Benzoic acid formula | C7H6O2 |
| Bicarbonate formula | CHO3– |
| Bleach formula | NaClO |
| Bleaching Powder Formula | CaOCl2 |
| Boric Acid formula | H3BO3 |
| Potassium Bromate formula | KBrO3 |
| Bromic acid formula | HBrO3 |
| Bromine formula | Br |
| Butane formula | C4H10 |
| Butanoic Acid formula | C4H8O2 |
| Calcium Acetate formula | C₄H₆CaO₄ |
| Calcium Bromide formula | CaBr2 |
| Calcium Carbonate formula | CaCO3 |
| Calcium hydride formula | CaH2 |
| Calcium hydroxide formula | Ca(OH)2 |
| Calcium Iodide formula | CaI2 |
| Calcium Nitrate formula | Ca(NO3)2 |
| Calcium Oxide formula | CaO |
| Carbon Monoxide formula | CO |
| Carbon Dioxide | CO2 |
| Carbon Tetrachloride formula | CCl4 |
| Carbonic Acid formula | H2CO3 |
| Calcium phosphate formula | Ca3(PO4)2 |
| Carbonic acid formula | H2CO3 |
| Citric acid formula | C6H8O7 |
| Chlorate formula | ClO–3 |
| Chlorine formula | Cl |
| Chlorine gas formula | Cl2 |
| Chlorous acid formula | HClO2 |
| Chromate formula | CrO42- |
| Chromic acid formula | H2CrO4 |
| Citric acid formula | C6H8O7 |
| Copper ii carbonate formula | CuCO3 |
| Copper ii nitrate formula | Cu(NO3)2 |
| Cyanide formula | CN– |
| Dichromate formula | K2Cr2O7 |
| Dihydrogen monoxide formula | H2O |
| Dinitrogen monoxide formula | N2O |
| Dinitrogen pentoxide formula | N2O5 |
| Dinitrogen trioxide formula | N2O3 |
| Ethanol formula | C2H5OH |
| Iron oxide formula | Fe2O3 |
| Ethene formula | C2H4 |
| Ethane | C2H6 |
| Ethylene glycol formula | C2H6O2 |
| Fluorine gas formula | F2 |
| Aluminum bromide formula | AlBr3 |
| Aluminum sulfide formula | Al2S3 |
| Ammonium carbonate formula | (NH4)2CO3 |
| Ammonium nitrate formula | (NH4)(NO3) |
| Ammonium phosphate formula | (NH4)3PO4 |
| Barium chloride formula | BaCl2 |
| Barium sulfate formula | BaSO4 |
| Calcium nitrate formula | Ca(NO3)2 |
| Carbon monoxide formula | CO |
| Carbon tetrachloride formula | CCl4 |
| Carbonic acid formula | H2CO3 |
| Hydrofluoric acid formula | HF |
| Hydroiodic acid formula | HI |
| Hypochlorous acid formula | HClO |
| Lithium phosphate formula | Li3PO4 |
| Magnesium nitrate formula | MgNO3 |
| Magnesium phosphate formula | Mg3(PO4)2 |
| Nitrogen monoxide formula | NO |
| Nitrous acid formula | HNO2 |
| Potassium carbonate formula | K2CO3 |
| Potassium iodide formula | KI |
| Potassium nitrate formula | KNO3 |
| Potassium phosphate formula | KH2PO4 |
| Sodium carbonate formula | Na2CO3 |
| Sodium oxide formula | Na2O |
| Fructose chemical formula (Glucose) | C6H12O6 |
| Glycerol formula | C3H8O3 |
| Helium gas formula | He |
| Hexane formula | C6H14 |
| Hydrobromic acid formula | HBr |
| Hydrochloric acid formula | HCl |
| Hydrocyanic acid formula | HCN |
| Hydrofluoric acid formula | HF |
| Hydrogen carbonate formula | CHO3– |
| Hydrogen gas formula | H2 |
| Hydrogen peroxide formula | H2O2 |
| Hydrogen phosphate formula | H3PO4 |
| Hydrogen sulfate formula | HSO4– |
| Hydrogen Sulfide | H2S |
| Hydroiodic acid formula | HI |
| Hydrosulfuric acid formula | H2SO4 |
| Hydroxide ion formula | OH– |
| Hypobromous acid formula | HBrO |
| Hypochlorite formula | NaClO |
| Hypochlorous acid formula | HClO |
| Hypoiodous acid formula | HIO |
| Iodic acid formula | HIO3 |
| Iodide ion formula | I– |
| Iodine formula | I2 |
| Iron (iii) nitrate formula | Fe(NO3)3 |
| Iron (ii) oxide formula | FeO |
| Iron (iii) carbonate formula | Fe2(CO3)3 |
| Iron (iii) hydroxide formula | Fe(OH)3 |
| Iron (iii) oxide formula | Fe2O3 |
| Iron (iii) chloride formula | FeCl3 |
| Lactic acid formula | C3H6O3 |
| Lead acetate formula | Pb(C2H3O2)2 |
| Lead (ii) acetate formula | Pb(C2H3O2)2 |
| Lead iodide formula | PbI2 |
| Lead (iv) oxide formula | PbO2 |
| Lead nitrate formula | Pb(NO3)2 |
| Lithium bromide formula | LiBr |
| Lithium chloride formula | LiCl |
| Lithium hydroxide formula | LiOH |
| Lithium iodide formula | LiI |
| Lithium oxide formula | Li2O |
| Lithium phosphate formula | Li3PO4 |
| Magnesium acetate formula | Mg(CH3COO)2 |
| Magnesium bicarbonate formula | C2H2MgO6 |
| Magnesium carbonate formula | MgCO3 |
| Magnesium chloride formula | MgCl2 |
| Magnesium hydroxide formula | Mg(OH)2 |
| Magnesium iodide formula | MgI2 |
| Magnesium nitrate formula | Mg(NO3)2 |
| Magnesium nitride formula | Mg3N2 |
| Magnesium carbonate formula | MgCO3 |
| Magnesium bromide formula | MgBr2 |
| Magnesium oxide formula | MgO |
| Magnesium phosphate formula | Mg3(PO4)2 |
| Magnesium sulfate formula | MgSO4 |
| Magnesium sulfide formula | MgS |
| Methane formula | CH4 |
| Methanol formula | CH3OH |
| Nickel acetate formula | Ni(C2H3O2)2 |
| Nickel nitrate formula | Ni(NO3)2 |
| Nitric acid formula | HNO3 |
| Nitride formula | N3– |
| Nitrite formula | NO2− |
| Nitrogen dioxide formula | NO2 |
| Nitrogen Gas | N2 |
| Nitrogen monoxide formula | NO |
| Nitrous acid formula | HNO2 |
| Oxalate formula | C2O42− |
| Oxalic acid formula | H2C2O4 |
| Oxygen Formula | O2 |
| Ozone formula | O3 |
| Perbromic acid formula | HBrO4 |
| Potassium Permanganate formula | KMnO4 |
| Permanganate ion formula | MnO4– |
| Phosphate formula | PO43- |
| Sodium hydrogen phosphate formula | Na2HPO4 |
| Sodium formate formula | HCOONa |
| Phenol formula | C6H6O |
| Phosphoric acid formula | H3PO4 |
| Phosphorus pentachloride formula | PCl5 |
| Phosphorus trichloride formula | PCl3 |
| Potassium acetate formula | CH3CO2K |
| Potassium bicarbonate formula | KHCO3 |
| Potassium carbonate formula | K2CO3 |
| Potassium chlorate formula | KClO3 |
| Potassium hydrogen phosphate formula | K2HPO4 |
| Potassium chloride formula | KCl |
| Potassium chromate formula | CrK2O4 |
| Potassium cyanide formula | KCN |
| Potassium dichromate formula | K2Cr2O7 |
| Potassium fluoride formula | KF |
| Potassium hydroxide formula | KOH |
| Potassium hypochlorite formula | KClO |
| Potassium iodide formula | KI |
| Potassium dihydrogen phosphate formula | KH2PO4 |
| Potassium nitrate formula | KNO3 |
| Potassium nitrite formula | KNO2 |
| Potassium oxide formula | K2O |
| Potassium iodate formula | KIO3 |
| Potassium phosphate formula | KH2PO4 |
| Potassium sulfite formula | K2SO3 |
| Salicylic acid formula | C7H6O3 |
| Silicon dioxide formula | SiO2 |
| Silver acetate formula | AgC2H3O2 |
| Silver carbonate formula | Ag2CO3 |
| Silver chloride formula | AgCl |
| Silver nitrate formula | AgNO3 |
| Silver oxide formula | Ag2O |
| Silver phosphate formula | Ag3PO4 |
| Sodium acetate formula | C2H3NaO2 |
| Sodium Bicarbonate (Baking Soda Formula) | NaHCO3 |
| Sodium bromide formula | NaBr |
| Sodium thiosulfate formula | Na2S2O3 |
| Sodium carbonate formula | Na2CO3 |
| Sodium chloride formula | NaCl |
| Sodium chromate formula | Na2CrO4 |
| Sodium citrate formula | Na3C6H5O7 |
| Sodium cyanide formula | NaCN |
| Sodium dichromate formula | Na2Cr2O7 |
| Sodium fluoride formula | NaF |
| Sodium hydroxide formula | NaOH |
| Sodium hypochlorite formula | NaClO |
| Sodium iodide formula | NaI |
| Uric acid formula | C5H4N4O3 |
| Sodium nitrate formula | NaNO3 |
| Sodium nitride formula | Na3N |
| Sodium nitrite formula | NaNO2 |
| Sodium oxide formula | Na2O |
| Sodium peroxide formula | Na2O2 |
| Sodium phosphate formula | Na3PO4 |
| Sodium sulfate formula | Na2SO4 |
| Sodium sulfide formula | Na2S |
| Sodium sulfite formula | Na2SO3 |
| Strontium chloride formula | SrCl2 |
| Strontium nitrate formula | Sr(NO3)2 |
| Sucrose formula | C12H22O11 |
| Sugar Formula | C12H22O11 |
| Sulfate ion formula | SO42− |
| Sulfur dioxide formula | SO2 |
| Sulfur trioxide formula | SO3 |
| Sulfuric acid formula | H2SO4 |
| Sulfurous acid formula | H2SO3 |
| Tartaric acid formula | C4H6O6 |
| Toluene formula | C7H8 |
| Urea formula | CH4N2O |
| Vinegar formula | C2H4O2 |
| Water Formula | H2O |
| Zinc acetate formula | Zn(O2CCH3)2 |
| Zinc carbonate formula | ZnCO3 |
| Zinc chloride formula | ZnCl2 |
| Zinc hydroxide formula | Zn(OH)2 |
| Zinc iodide formula | ZnI2 |
| Zinc nitrate formula | Zn(NO3)2 |
| Zinc phosphate formula | Zn3(PO4)2 |
| Zinc sulfate formula | ZnSO4 |
| Zinc sulfide formula | ZnS |
Chemical Formula List
Here we have discussed a list of 108 chemical formulas for various compounds.
- CH3COOH – Acetic Acid
- HC2H3O2 – Acetate Ion
- NO2 – Nitrogen Dioxide
- SO4²- – Sulfate Ion
- PO4³- – Phosphate Ion
- O3 – Ozone
- H2CO3 – Carbonic Acid
- Na2CO3 – Sodium Carbonate
- MgSO4 – Magnesium Sulfate
- KNO3 – Potassium Nitrate
- Ca(OH)2 – Calcium Hydroxide
- FeSO4 – Iron(II) Sulfate
- Al2O3 – Aluminum Oxide
- NaHCO3 – Sodium Bicarbonate (Baking Soda)
- C6H5COOH – Benzoic Acid
- C4H10 – Butane
- C3H8 – Propane
- C2H2 – Ethyne (Acetylene)
- H2SiO3 – Silicic Acid
- H2PO4^- – Dihydrogen Phosphate Ion
- C2H3Cl – Vinyl Chloride
- C3H6O – Acetone
- Na2SO4 – Sodium Sulfate
- KMnO4 – Potassium Permanganate
- NaNO3 – Sodium Nitrate
- FeCl3 – Iron(III) Chloride
- NH4OH – Ammonium Hydroxide
- C6H4OH – Hydroquinone
- H2C2O4 – Oxalic Acid
- C6H5CH3 – Toluene
- C7H6O2 – Salicylic Acid
- C7H6O3 – Aspirin
- C8H10 – Xylene
- C6H5NH2 – Aniline
- C5H5N – Pyridine
- C6H5NO2 – Nitrobenzene
- H3PO4 – Phosphoric Acid
- Ca3(PO4)2 – Calcium Phosphate
- FeS – Iron(II) Sulfide
- NaClO – Sodium Hypochlorite
- CH3COCH3 – Acetophenone
- NaCN – Sodium Cyanide
- C6H5CHO – Benzaldehyde
- C6H5COCH3 – Acetophenone
- C2H4O2 – Acetic Anhydride
- CH3CH2COCH3 – Butanone (Methyl Ethyl Ketone)
- C6H5COCH2C6H5 – Acetylbenzene (Acetophenone)
- C6H5COOH – Benzoic Acid
- C6H5COC6H5 – Benzophenone
- C10H14N2 – Nicotine
- C6H5COC6H5 – Benzil
- C10H12O2 – Vanillin
- C10H14N2O – Caffeine
- C9H8O4 – Aspirin
- C12H10O4 – Terephthalic Acid
- C14H8O4 – Anthraquinone
- C12H8O4 – Resorcinol
- C15H10O4 – Hydroquinone
- C12H9N – Quinoline
- C10H7N – Indole
- C9H6N2O2 – Barbituric Acid
- C7H5N3O6 – TNT (Trinitrotoluene)
- C6H2(NO2)3CH3 – Tetryl
- C7H5N5 – Caffeine
- C6H5N3O9 – Nitroglycerin
- C6H12N2O4S2 – Thiamine (Vitamin B1)
- C6H8O6 – Ascorbic Acid (Vitamin C)
- C12H17N4O4S – Coenzyme A
- C10H16N2O3S – Acetyl-CoA
- C21H28O2 – Progesterone
- C21H30O5 – Cortisol
- C21H20O6 – Estrone
- C22H32O2 – Testosterone
- C19H28O2 – Prostaglandin E2
- C24H30O4 – Cholesterol
- C5H11NO2S – Cysteine
- C6H13NO2 – Arginine
- C5H9NO4 – Glutamine
- C6H13NO5 – Asparagine
- C9H11NO3 – Tyrosine
- C5H9NO4 – Aspartic Acid
- C3H7NO2 – Serine
Importance of Chemical Formula
Chemical formulas are the cornerstone of chemistry, playing a pivotal role in understanding, communicating, and manipulating the properties and behavior of matter.
- Identification: Chemical formulas uniquely identify substances. They specify the types and quantities of atoms in a compound, allowing scientists to distinguish one substance from another.
- Communication: Formulas are a universal language in chemistry. Scientists worldwide use them to convey information about compounds, facilitating effective communication and collaboration.
- Stoichiometry: Formulas help determine the stoichiometry of chemical reactions, which is crucial for calculating reactant and product quantities, as well as understanding reaction mechanisms.
- Predicting Properties: The chemical formula provides insights into the physical and chemical properties of compounds, such as their melting points, boiling points, and reactivity.
- Safety: Understanding the chemical formulas of substances is vital for safety in laboratories and industries. It helps in the handling, storage, and disposal of chemicals.
- Education: Learning chemical formulas is a foundational aspect of chemistry education, enabling students to grasp the fundamental principles of the field.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













