The Central Board of Secondary Education has released the CBSE Class 12 Chemistry Additional Practice Question Paper 2025-26 for all Class 12th students who have chosen science as their major stream. The students can download their Class 12 practice Paper 2025-26 (CBSE) by visiting the official website i.e., www.cbseacademic.nic.in. Students preparing to appear in the CBSE class 12th Chemistry exam are advised to download and practice the CBSE Chemistry Sample Questions Paper 2025-26. As these practice papers will help them to understand and familiarize themselves with the newly updated format of the Class 12th Board exams. Students can click on the direct PDF link provided below to download the entire CBSE Class 12th Chemistry Practice Questions Paper 2024-25.
CBSE Class 12 Chemistry Practice Question Paper 2025-26
CBSE Board has also released the blueprint and marking scheme for the Class 12 Chemistry Board Exam 2025-26. The most interesting fact about the updated CBSE Class 12 exam pattern is that it will consist of 50% of competency questions, which might help the students in their further studies too. The question paper of the class 12 chemistry exam is divided into five sections with 35 questions for 70 marks.
The CBSE Class 12th Sample Papers 2025-26 are a type of practice test designed by the Central Board of Secondary Education to help students prepare for their board exams. Students can use the Class 12th Chemistry sample paper 2025-26 as a tool to assess their understanding of the chemistry syllabus and enhance their overall exam readiness.
CBSE Class 12 Chemistry Practice Paper 2025-26 with Solutions
The CBSE Sample Paper for Class 12th Chemistry has a similar paper pattern to the CBSE Class 12th Chemistry Question Paper 2024-25. The Chemistry sample paper includes questions from different sections, like physical, organic, and inorganic chemistry. The total marks for the CBSE class 12 Chemistry exam will be 100 marks out of which the theory paper will be 70 marks while the remaining 30 marks will be provided for the Chemistry Practical exam 2025-26.
| CBSE Class 12th Chemistry Sample Paper 2025-26 | |
| Exam Conducting Body | Central Board of Secondary Education |
| Name of the Examination | CBSE Class 12th Board Examination 2025-26 |
| Category | Sample Paper |
| Status | Released |
| Mode of Sample Paper Availability | Online Mode |
| Official Website | www.cbseacademic.nic.in |
Class 12 Chemistry Practice Paper 2025-26
The CBSE Class 12 Chemistry Sample Model Questions Paper 2025-26 is also designed from a board exam point of view, which is similar to your board exam pattern. The complete exam pattern that has been followed is as follows-
There are 33 questions, which are divided into 5 sections, in the Class 12 chemistry question paper with internal choice.
(b) SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
(c) SECTION B consists of 5 short-answer questions carrying 2 marks each.
(d) SECTION C consists of 7 short-answer questions carrying 3 marks each.
(e) SECTION D consists of 2 case-based questions carrying 4 marks each.
(f) SECTION E consists of 3 long answer questions carrying 5 marks each.
(h) Use of log tables and calculators is not allowed.
Chemistry Class 12 Additional Practice Question Paper 2025-26 PDF with Solutions
All the students who are going to appear for the CBSE Class 12th Chemistry Board Exam 2024-25 must prepare well. Practice through the latest CBSE Class 12th Chemistry Sample Paper 2025-26 PDF is one of the best ways of revision before going for the exam. The students who are revising their Class 12 Chemistry topics for their upcoming CBSE Class 12 Chemistry Board Exam should download the official Practice question paper PDF along with its solution from the link below. We have provided the complete Chemistry Class 12 Sample Paper 2025-26 Download link and the solutions PDF link for the convenience of candidates.
| CBSE Class 12 Chemistry Additional Practice Question Paper 2025-26 PDF with Solutions | |
| Sample Paper | Solution PDF |
| Chemistry Class 12 Sample Paper 1 2024-25 | Solution Link |
| Chemistry Class 12 Sample Paper 2 2024-25 | |
CBSE Class 12 Chemistry Additional Practice Question Paper 2025-26 with Solutions
Science stream students who are preparing for their board exams should take the opportunity to attempt to solve these questions by themselves and cross-check with the solutions provided to score well in their real-time exams.
Q1. Sunita set up three cells as shown below:
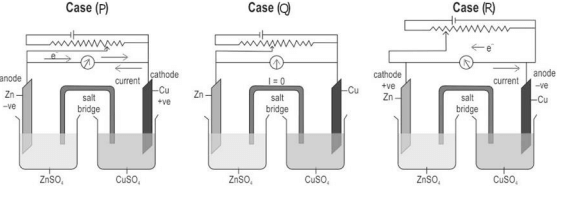
She applied external potential in all three cells. The potential is increased slowly, till the opposing voltage reaches the value of 1.1 V.
Which of the following statements is INCORRECT?
(a) Electrons flow from the Zn rod to the Cu rod hence current flows from Cu to Zn in case (P).
(b) The chemical reaction takes place in case (Q) till the opposing voltage reaches 1.1 V.
(c) Zinc is deposited at the zinc electrode and copper dissolves at the copper electrode in case (P).
(d) Electrons flow from Cu to Zn and current flows from Zn to Cu in case (R).
Ans. (c) Zinc is deposited at the zinc electrode and copper dissolves at the copper electrode in case (P).
Q2. Two compounds M and N have the general formula CnH2nO but different structural formulae.
i) Compound N belongs to that homologous series where the first member contains 3
carbon atoms.
ii) Compound M reacts with one equivalent of monohydric alcohol in the presence of
dry hydrogen chloride to yield a hemiacetal.
Identify the homologous series to which compounds M and N belong to.
(a) Both the compounds are aldehydes.
(b) Compound M is an aldehyde and compound N is a ketone.
(c) Both the compounds are ketones.
(d) Compound N is an aldehyde and compound M is a ketone.
Ans. (b) Compound M is an aldehyde and compound N is a ketone.
Q3. What will be the change in the hybridization of C when a nucleophile attacks the electrophilic center of the carbonyl group?
(a) sp2 to sp
(b) sp3 to sp2
(c) sp3 to sp
(d) sp2 to sp3
Ans. (d) sp2 to sp3
Q4. Four compounds, CH3Cl, CH3Br, C2H5Br, and C3H7I are represented by the letters M, N, O, and P in the table below (in random order). The boiling points are also given in the table.
| Boiling Points of various compounds | ||||
| Boiling points(BP) | -24.2°C | 38°C | 3.56°C | 101.6°C |
| Compound | M | N | O | P |
Which of the four compounds does ‘N’ most likely represent?
(a) CH3Cl
(b) CH3Br
(c) C2H5Br
(d) C3H7I
Ans. (c) C2H5Br
Q5. Sampriti took 4 acids. Help her to arrange the acids from left to right, in the increasing
order of their acidity:
2, 4, 6 – Trinitrophenol, acetic acid, phenol, and benzoic acid.
(a) 2, 4, 6 – Trinitrophenol, acetic acid, benzoic acid, phenol
(b) phenol, acetic acid, benzoic acid, 2, 4, 6 – Trinitrophenol
(c) 2, 4, 6 – Trinitrophenol, benzoic acid, acetic acid, phenol
(d) phenol, benzoic acid, acetic acid, 2, 4, 6 – Trinitrophenol
Ans. (b) phenol, acetic acid, benzoic acid, 2, 4, 6 – Trinitrophenol
Q6. Study the graph given below.
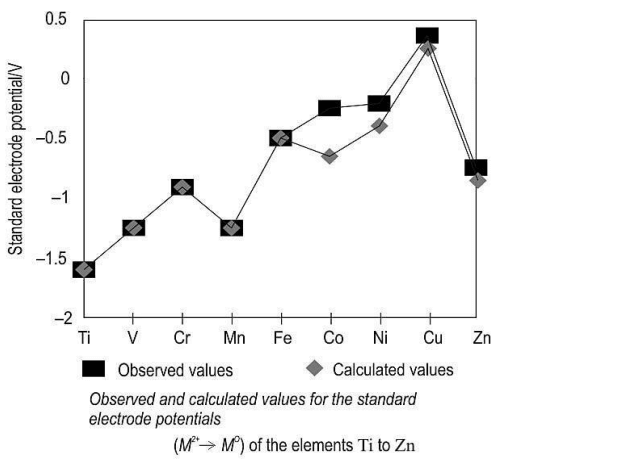
Based on the graph given, which element will MOST LIKELY be involved in the following reaction?
Metal + conc. sulphuric acid → Metal sulphate + sulphur dioxide + water
(a) Cu
(b) Co
(c) Ti
(d) Zn
Ans: (a) Cu
Q7. The table given below shows the results of three experiments on the rate of the reaction between compounds P and Q at a constant temperature.
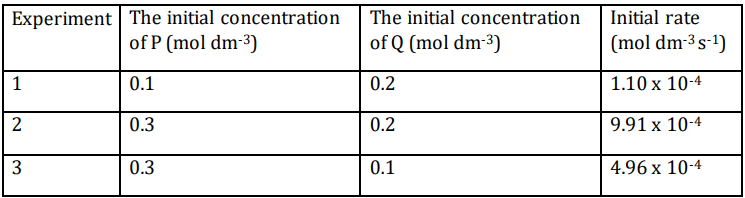
Based on the data, what will be the rate equation for the reaction between P and Q?
(a) k[P]²[Q]
(b) k[P][Q]²
(c) k[P][Q]
(d) k[P]
Ans: (a) k[P]²[Q]
Q8. The table below shows the KH values for some gasses at 293 K and at the same pressure.
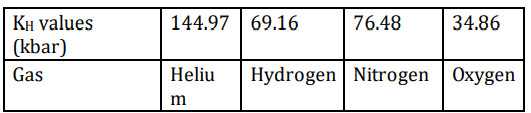
In which of the following are the gases arranged in their decreasing order of solubility (from left to right)?
(a) Helium > Nitrogen > Hydrogen > Oxygen
(b) Hydrogen > Helium > Nitrogen > Oxygen
(c) Nitrogen > Hydrogen > Oxygen > Helium
(d) Oxygen > Hydrogen > Nitrogen > Helium
Ans: (d) Oxygen > Hydrogen > Nitrogen > Helium
Q9. Sampriti took 4 acids. Help her to arrange the acids from left to right, in the increasing
order of their acidity: 2, 4, 6 – Trinitrophenol, acetic acid, phenol, and benzoic acid.
(a) 2, 4, 6 – Trinitrophenol, acetic acid, benzoic acid, phenol
(b) phenol, acetic acid, benzoic acid, 2, 4, 6 – Trinitrophenol
(c) 2, 4, 6 – Trinitrophenol, benzoic acid, acetic acid, phenol
(d) phenol, benzoic acid, acetic acid, 2, 4, 6 – Trinitrophenol
Ans: (b) phenol, acetic acid, benzoic acid, 2, 4, 6 – Trinitrophenol
Q10. An archeologist found that the percentage of carbon-14 in a wooden artifact was 20% of what carbon-14 would have been in the wood when it was cut from the tree.
What would be the approximate age of this wooden artifact?
(Given the half-life of carbon-14= 5730 years)
(a) 5,790 years
(b) 12,060 years
(c) 13,300 years
(d) 38,000 years
Ans: (c) 13,300 years



 CBSE Class 12 Business Studies Sample Qu...
CBSE Class 12 Business Studies Sample Qu...
 CBSE Class 12 Physics Model Paper 2024-2...
CBSE Class 12 Physics Model Paper 2024-2...













