Define Semiconductors
Semiconductors are materials that fall between conductors (like metals) and insulators (like rubber) in terms of electrical conductivity. They have a unique property that says that “their conductivity can be altered, allowing them to switch between being good conductors and insulators”. This property is very important for building electronic devices. Silicon is the most common semiconductor material. In semiconductors, the flow of electrical current can be controlled, enabling the creation of electronic components like transistors and diodes.
Transistors act as tiny switches, controlling the flow of electrons in electronic circuits. This controllability is fundamental for building complex devices like computers, smartphones, and more. The ability to regulate electrical flow makes semiconductors the backbone of modern electronics, facilitating the manipulation and processing of information in a variety of electronic devices.
Energy Band Theory of Semiconductors
The energy band theory describes the arrangement of electrons in materials like semiconductors. It categorizes electron energy levels into bands namely: the Valance Band, where electrons are tightly bound; and the Conductive Band, where electrons can move freely. In semiconductors, there’s a small energy gap between these bands. Electrons in the valance bands need a boost of energy to jump to the conduction band, creating an electron-hole pair.
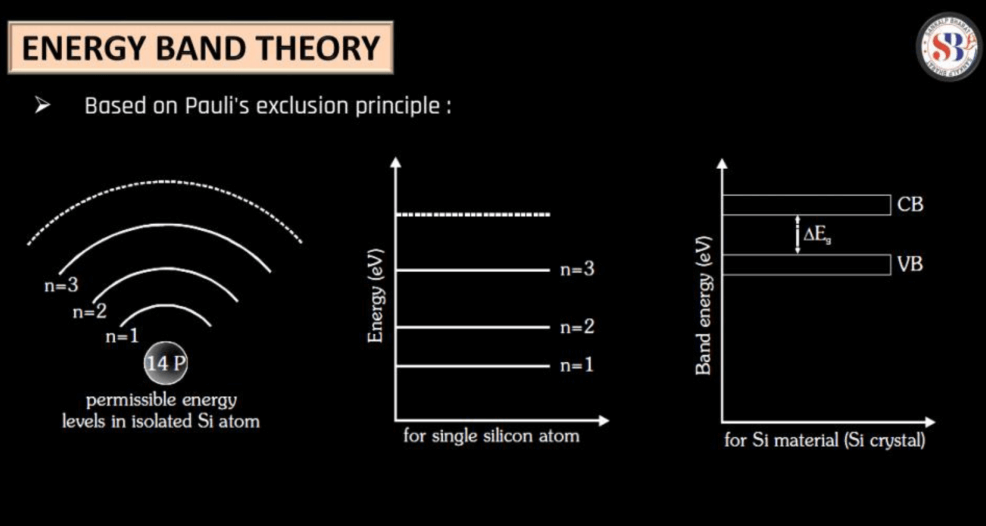
This energy gap determines a material’s conductivity. Semiconductors can conduct electricity under certain conditions, like when exposed to light or heat. Doping, adding impurities, can also modify their properties. This theory helps explain how semiconductors function in electronic devices, playing a crucial role in technologies like transistors and integrated circuits.
| Different Energy Bands | |
| Energy Bands | Description |
| Valance Band | In valance bands electrons are tightly bound to atoms. These bands determine the electrical and optical properties and contribute to the material’s stability. |
| Conduction Band | In Conduction bands electrons can move freely, facilitating conductivity. These bands contain electrons with higher energy. And determines the material’s ability to conduct electricity. |
Concept of “Holes” in Semiconductors
In semiconductors, like silicon, electrons carry an electrical charge. However, the concept of “holes” helps us understand their behavior. Think of the absence of an electron as a positive charge, creating a virtual charge carrier called a hole. When an electron leaves its position, it leaves behind a hole that can move through the crystal lattice. Now, picture this like a game of musical chairs: an electron (player) moves, leaving an empty chair (hole) behind. The movement of these holes contributes to electric current flow.
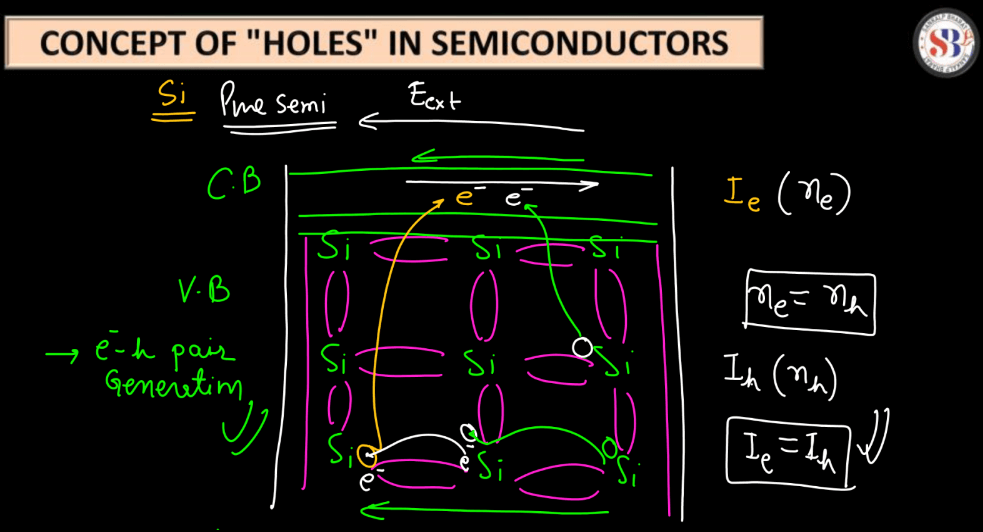
Interestingly, it’s easier to imagine that holes moving than tracking individual electrons. In semiconductors, both electrons and holes participate in current flow, but understanding holes simplifies analysis. Engineers manipulate these electron-hole pairs to design electronic devices like transistors. So, the concept of holes is a clever abstraction aiding our comprehension of semiconductor behavior and enabling the creation of various electronic gadgets.
Band Gaps or Forbidden Energy Gap
The band gaps also known as forbidden energy gaps, are a crucial concept in understanding the behaviour of electrons in materials, particularly in semiconductors. Here we have mentioned a few points that will help you understand Band gaps more clearly:
- Energy Levels: Imagine energy levels of electrons in a material like steps on a staircase. Electrons can occupy these steps, representing different energy states.
- Valance Band and Conduction Band: The steps can be divided into two main regions – the lower one is called the valance band, where electrons normally reside, and the upper one is the conduction band, where electrons can move freely and conduct electricity.
- Band Gap: Between the valence and conduction bands, there exists a gap, known as the band gap. This gap represents energy levels that electrons cannot occupy under normal conditions.
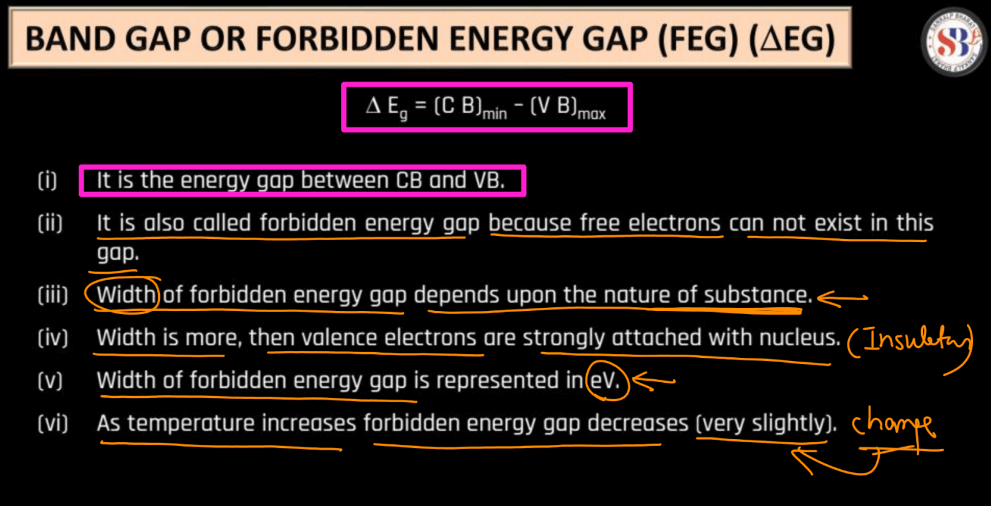
- Electron Movement: For an electron to move from the valence band to the conduction band (to conduct electricity), it needs to acquire energy equal to or greater than the band gap.
- Conductors, Semiconductors, and Insulators: Materials with a small or no band gap are conductors because electrons can easily move between bands. Semiconductors have a moderate band gap, providing a controllable level of conductivity. Insulators have a large band gap, making it difficult for electrons to move, and thus, they don’t conduct well.
- Electronic Devices: Engineers manipulate the band gap in semiconductor materials to design electronic devices like transistors and diodes, enabling the precise control of electron flow and the creation of various electronic components.
Types of Semiconductor
Semiconductors are classified into two main types namely: Intrinsic semiconductors and Extrinsic semiconductors. Where intrinsic semiconductors are pure semiconductors, like silicon or germanium, without any intentional impurity while in Extrinsic semiconductors doping is involved in intentionally adding impurities to semiconductors to alter their electrical properties.
| Different Types of Semiconductors | |
| Types | Description |
| Intrinsic semiconductors | The intrinsic semiconductors are pure semiconductors without intentional impurities. |
| Extrinsic semiconductors | Extrinsic semiconductors involve intentional impurities. Extrinsic semiconductors are of two types N-type and P-type. |
Intrinsic semiconductors
- Intrinsic semiconductors are pure semiconductor materials, such as silicon (Si) or Germanium (Ge), with no intentional impurity.
- At absolute zero temperature, they behave as insulators because electrons are tightly bound to atoms.
![]()
- As temperature increases, electrons gain energy, creating electron-hole pairs. These free electrons and holes contribute to electrical conductivity.
- The number of electrons and holes in intrinsic semiconductors is equal, resulting in a balance between positive and negative charge carriers.
Extrinsic Semiconductors
- Extrinsic semiconductors are intentionally modified by adding impurities through a process called doping.
- Doping allows precise control over the electrical properties of semiconductors, making them suitable for various electronic applications, including transistors.
- The extrinsic semiconductors are of two types N-types and P-types.
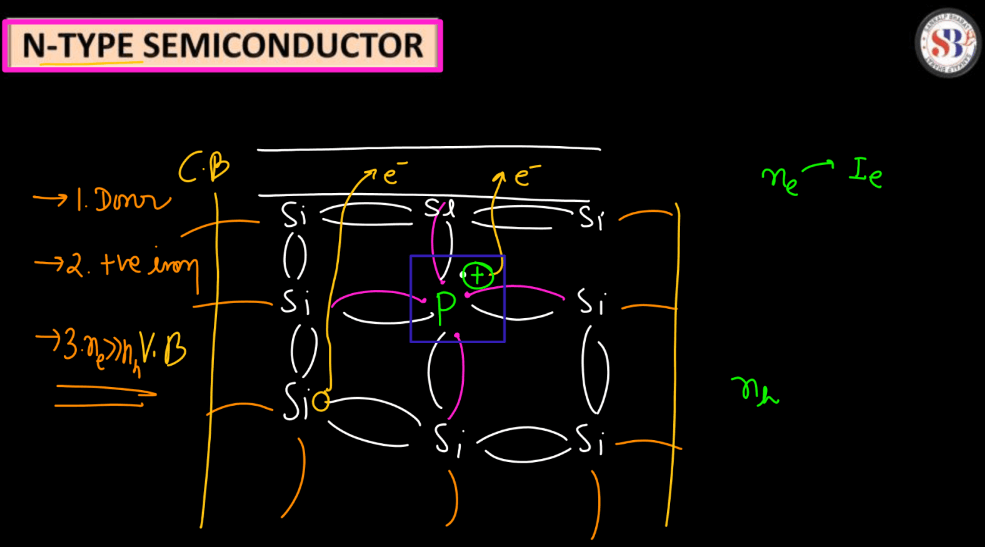
N-types (Negative-type)
- Negative semiconductors are doped with elements like phosphorus or arsenic, introducing extra electrons into the crystal lattice.
- Electrons become the majority charge carriers, leading to higher electron conductivity.
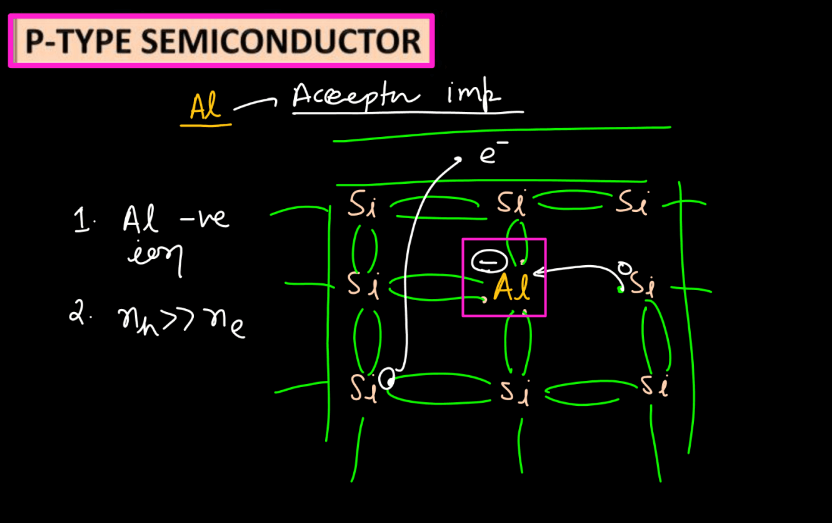
P-types (Positive-type)
- The Positive semiconductors are doped with elements like boron or aluminum, creating holes (missing electrons) in the crystal lattice.
- Holes become the majority charge carriers, contributing to hole conductivity.
Properties of Semiconductors
Semiconductors are materials with electrical conductivity between that of conductors and insulators. The semiconductors form the foundation of electronic devices, enabling the creation of transistors, diodes, and integrated circuits. Here we have mentioned a few points that will highlight the properties of semiconductors.
- Conductivity: Intermediate electrical conductivity compared to conductors and insulators.
- Band Gap: Semiconductors have a small energy gap between their valence and conduction bands, allowing for controlled electron movement.
- Doping: Adding impurities through doping can enhance conductivity. N-type doping adds electrons, while P-type doping adds “holes” or vacancies.
- Temperature Sensitivity: Conductivity increases with temperature due to more electrons moving to the conduction band.
- Mobility: Electron’s ability to move through a semiconductor is characterized by mobility, influencing its electrical performance.
- Optical Properties: Some semiconductors exhibit optical effects, like the ability to emit light when exposed to an electric current (as in LEDs).
- Thermal conductivity: It’s lower than metals but higher than insulators, influencing heat dissipation.


 Ohm's Law: Definition, Formula, Limitati...
Ohm's Law: Definition, Formula, Limitati...
 Newton's First Law of Motion: Definition...
Newton's First Law of Motion: Definition...
 Kepler's Laws of Planetary Motion: First...
Kepler's Laws of Planetary Motion: First...













