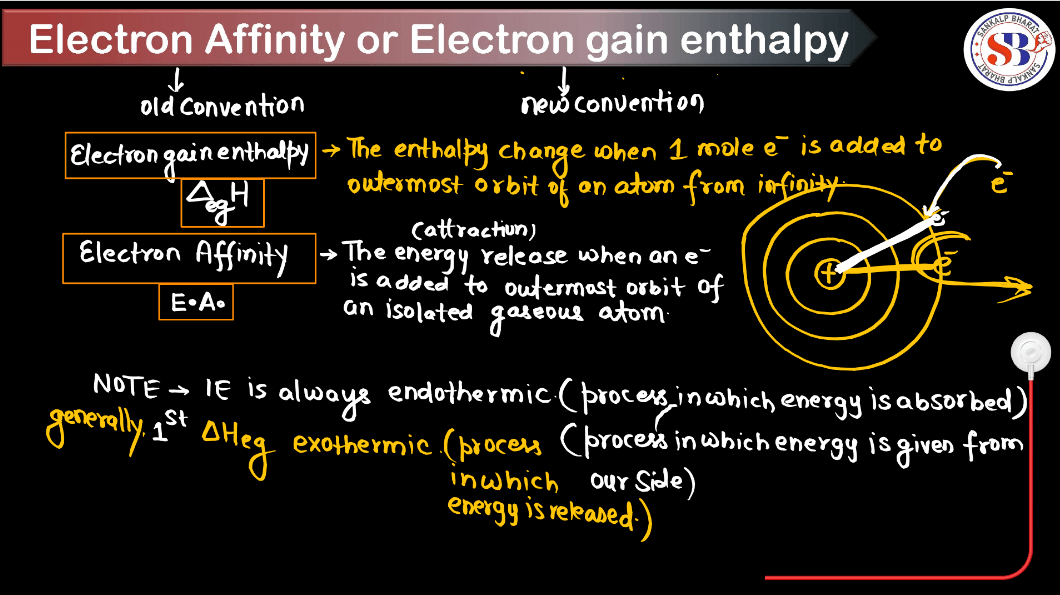
Define Electron Gain Enthalpy
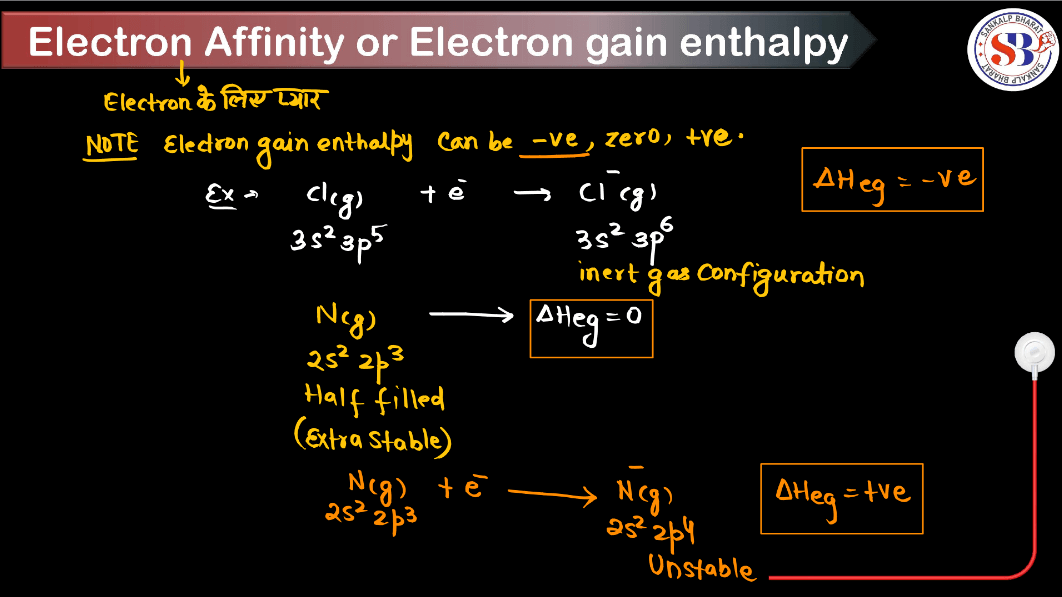
Electron gain enthalpy, in simple terms, describes the energy change that occurs when a neutral atom gains an electron to form a negative ion. It is a measure of how much energy is released or absorbed during this process. When an atom gains an electron, it usually releases energy, resulting in a negative enthalpy change, because the electron is attracted to the positively charged Nucleus. However, some atoms may require energy input to accept an electron due to factors like electron-electron repulsions. Electron gain enthalpy helps understand an atom’s tendency to gain electrons and form ions, which is crucial in chemistry for predicting reactivity and the formation of chemical bonds.
Electron gain enthalpy also known as electron affinity was developed as part of a broader understanding of atomic structure and chemical bonding. It is closely related to concepts in Thermodynamics and quantum mechanics. While it is challenging to attribute the discovery of electron gain enthalpy to a single individual, its development can be traced back to the works of various scientists in the late 19th and early 20th centuries. Electron gain enthalpy has become a fundamental concept in chemistry.
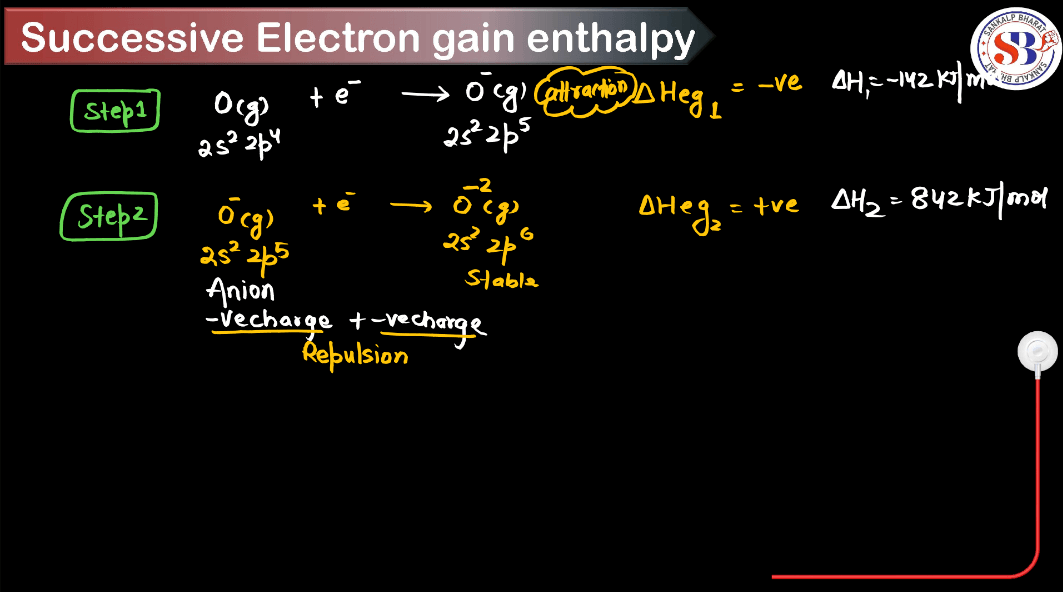
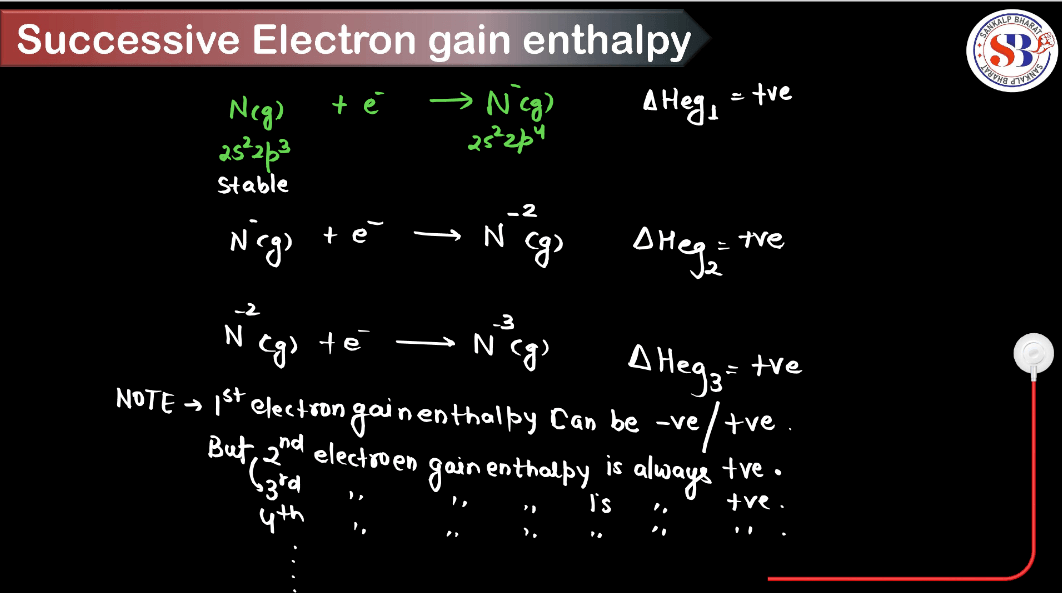
What is Successive Electron Gain Enthalpy?
Successive electron gain enthalpy is a measure of energy change when an atom gains an electron after already gaining one or more electrons. Imagine atoms as houses: the first electron is like a guest moving into an empty house, easily accommodated. But as more electrons are added, it’s like trying to cram more guests into a full house; it becomes increasingly difficult, requiring more energy. Successive electron gain enthalpy quantifies this energy increase. In simpler terms, it’s like squeezing more people into an already crowded space, demanding more effort. It reflects the atom’s reluctance to accept additional electrons due to increased repulsion among negatively charged electrons already present.
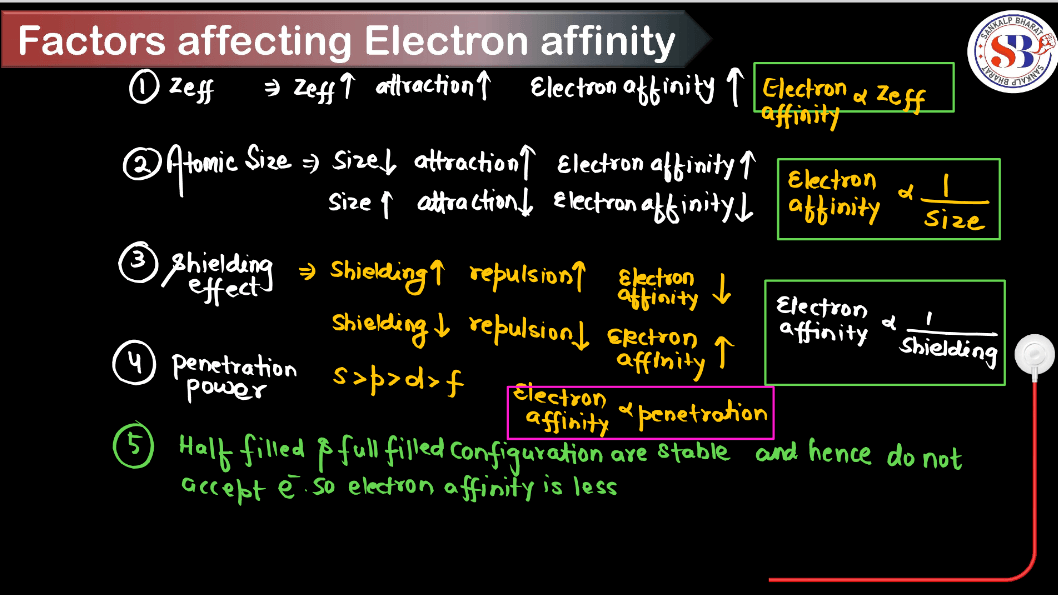
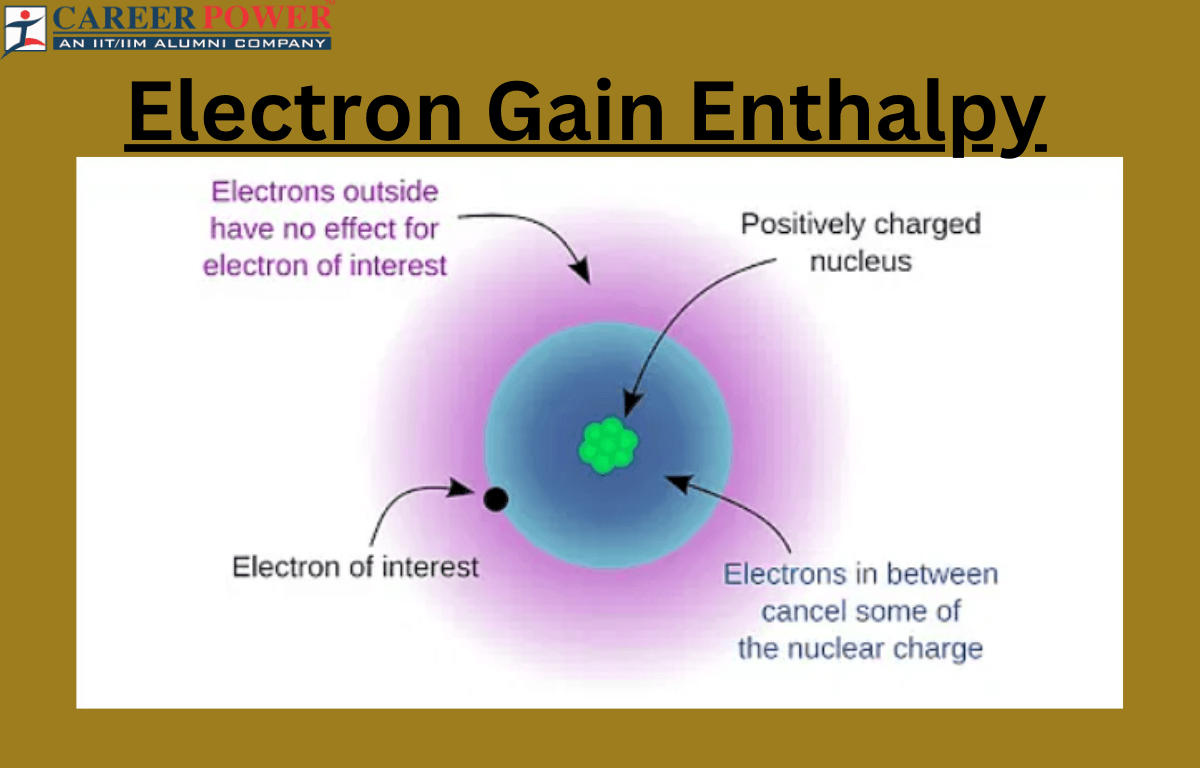
Factors Affecting Electron Gain Enthalpy
Electron Gain Enthalpy (EGH) is the energy released when an atom in the gas phase gains an electron to form a negative ion. Several factors influence the electron gain enthalpy:
- Atomic Size: Smaller atoms have higher electron gain enthalpy because the incoming electron experiences greater electrostatic attraction from the nucleus.
- Nuclear Charge: Higher nuclear charge increases electron gain enthalpy since the nucleus exerts a stronger pull on incoming electrons.
- Electron-Electron Repulsion: If the electron is added to an already negatively charged ion, there can be electron-electron repulsion, which can decrease the electron gain enthalpy.
- Electron Configuration: Half-filled or fully-filled orbitals tend to have lower electron gain enthalpy due to increased stability.
- Shielding Effect: Inner electrons shield the outer electrons from the full effect of the nuclear charge, reducing the electron gain enthalpy.
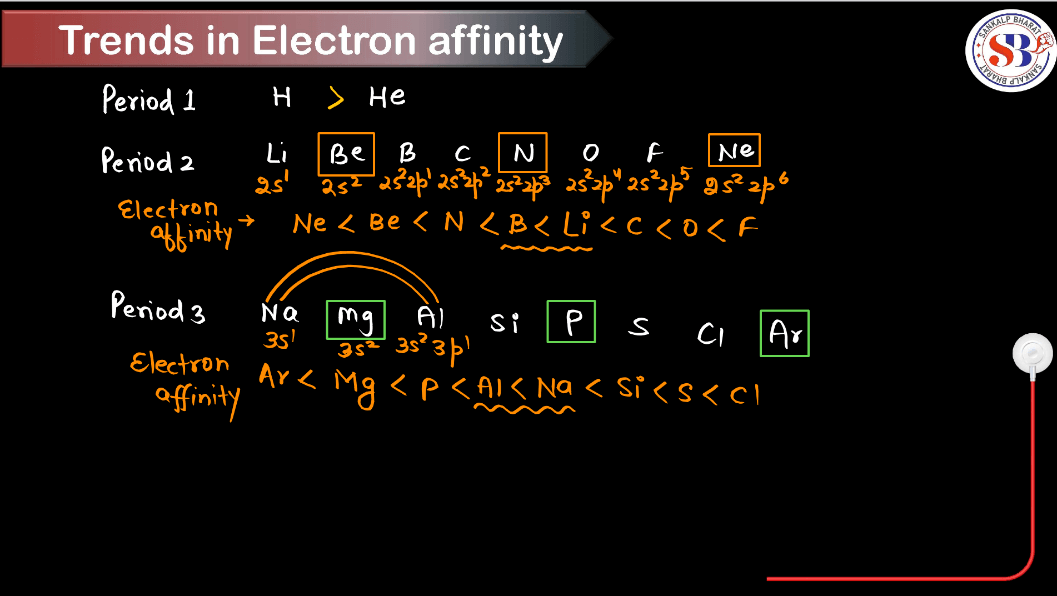
Trends of Electron Gain Enthalpy in the Periodic Table
Electron gain enthalpy (also known as Electron Affinity) is the energy released when an atom gains an electron. Understanding these trends helps predict the reactivity and chemical behavior of elements across the periodic table. The trends of electron gain enthalpy in the periodic table are as follows:
- Across a Period (Left to Right):
- Generally, electron gain enthalpy becomes more negative (exothermic) from left to right across a period.
- This trend is due to increasing effective nuclear charge, which pulls incoming electrons closer to the nucleus, resulting in higher energy releases.
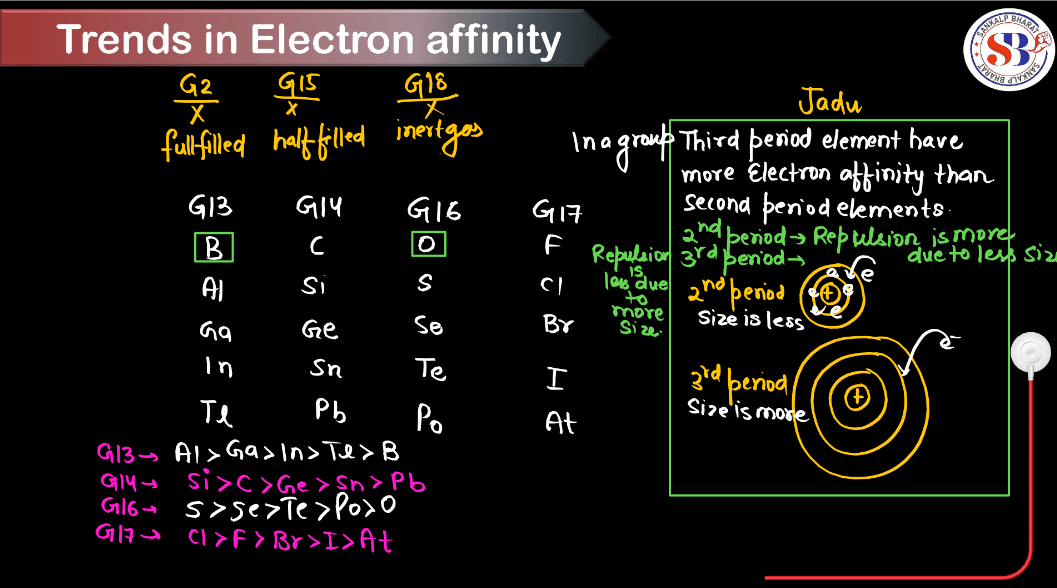
- Down the Group (Top to Bottom):
- Electron gain enthalpy becomes less negative (or even positive) down a group.
- This trend occurs because as you move down a group, the atomic size increases, leading to a decrease in the effective nuclear charge experienced by the outermost electrons. Therefore, it becomes easier to add an electron because the nucleus has less pull on the incoming electron.
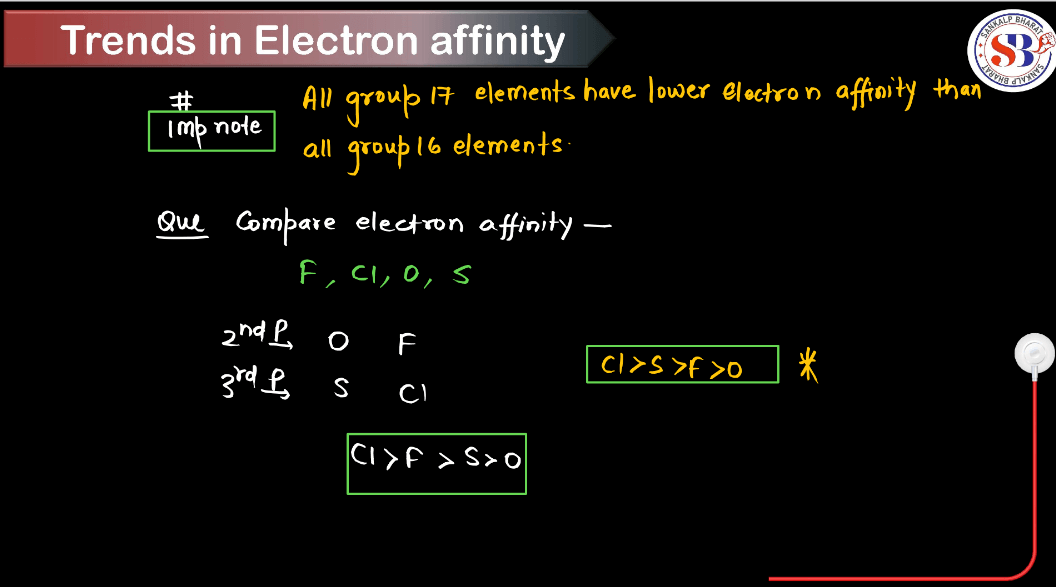
- Exceptions:
- There are some notable exceptions, particularly in elements of the p-block.
- For example, the electron gain enthalpy of oxygen is less negative than that of sulfur despite being in the same group. This is because adding an electron to oxygen would result in repulsion due to electron-electron interaction, making the process less energetically favorable.
- Another example is the noble gases, which have positive electron gain enthalpy because adding an electron configuration requires energy rather than releasing it.



 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













