Thermodynamics
Thermodynamics is the study of heat and energy transfer in physical systems. It explores how energy changes within a system, whether it’s a steam engine, a car engine, or even the human body. There are four main laws of thermodynamics that govern these energy transformations. The first law of thermodynamics, known as the conservation of energy, states that energy can neither be created nor destroyed, it can only be converted from one form to another. The Second Law of Thermodynamics introduces the concept of entropy, suggesting that natural processes tend to move towards disorder or increased randomness.
The third law of thermodynamics deals with absolute zero, the lowest possible temperature where entropy approaches a minimum value. Finally, the zeroth law deals establishes the concept of temperature and thermal equilibrium. Together these laws provide a framework for understanding and providing the behavior of systems undergoing changes in temperature, pressure, and energy. Thermodynamics is crucial in designing efficient engines, and refrigeration systems, and understanding fundamental processes in chemistry and physics.
Thermodynamic Terms
Thermodynamics involves various terms such as system, surroundings, and boundaries. These thermodynamic terms help in understanding and analyzing the behavior of physical systems, particularly in relation to energy and heat transfer. By employing these terms, scientists and engineers can formulate and solve thermodynamic problems, predict system behavior, and design efficient energy processes. They form the foundation for understanding and manipulating the principles governing energy and heat transfer in various systems.
| Thermodynamic Terms | |
| Terms | Description |
| System | A system is a specific region or substance under study, isolated from its surroundings, with energy and matter exchanges analyzed for understanding physical processes. |
| Surrounding | Surrounding is thermodynamics, which refers to everything outside the defined system, influencing it. Interactions between the system and surroundings play a crucial role in energy transfers and transformation. |
| Boundaries | Boundaries in thermodynamics delineate the limit of a system, separating it from the surroundings. These boundaries can be real or imaginary, influencing the exchange of energy and matter. |
System
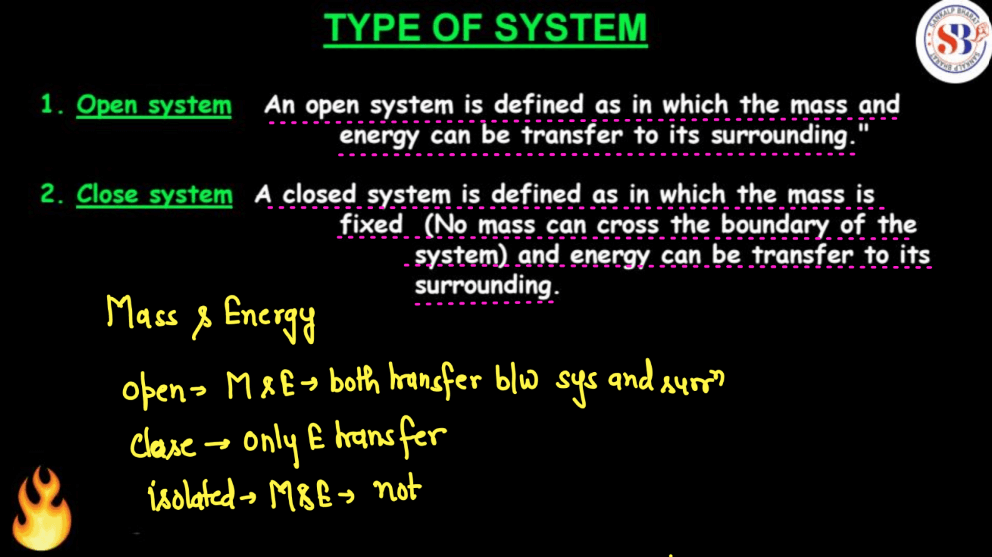
A system refers to a specific region or object under study. It can be as simple as a gas-filled balloon or as complex as an entire steam engine. Thermodynamics explores how energy transfers and transformations occur within these systems. There are three main types of systems namely, Isolated systems, Open systems, and Closed systems. Open system, where both energy and matter can be exchanged with the surroundings; and Closed system, which allows energy exchange but not matter. Understanding a system’s properties, such as temperature, pressure, and volume, helps analyze and predict changes in its state. Thermodynamics principles guide the study of energy flow and transformations within these systems, influencing everything from engines to natural processes
| Different Types of Systems | |
| Types | Description |
| Isolated System | An isolated system restricts both matter and energy transfer with its surroundings. Picture a sealed box, maintaining its content and preventing any exchange with the environment. |
| Open System | An open system is like a porous boundary; it allows interactions and exchanges with its surroundings, enabling the flow of matter and energy across its boundaries. |
| Closed System | A closed system is self-contained, with no exchange of matter but allows energy transfer. It’s like a sealed jar, conserving its contents while allowing heat or work interactions. |

Surroundings
Surroundings refer to everything outside a system under consideration. A thermodynamic system is a region of space that is chosen for study, and the surroundings include everything else. The system and its surroundings interact through the exchange of energy and matter. For example, if you’re studying a gas in a container (the system), the wall of the container and the air outside (surroundings) play a crucial role. Thermodynamics examines how energy transformations occur within a system and its surroundings. Understanding the relationship between a system and its surroundings is fundamental to analyzing heat transfer, work, and changes in the state of matter within the context of thermodynamics processes.
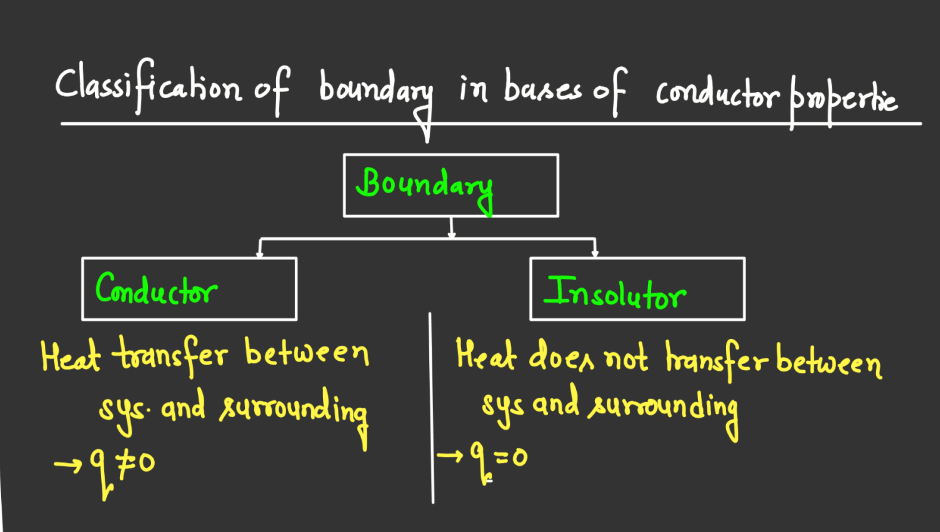
Boundaries
Thermodynamic Processes
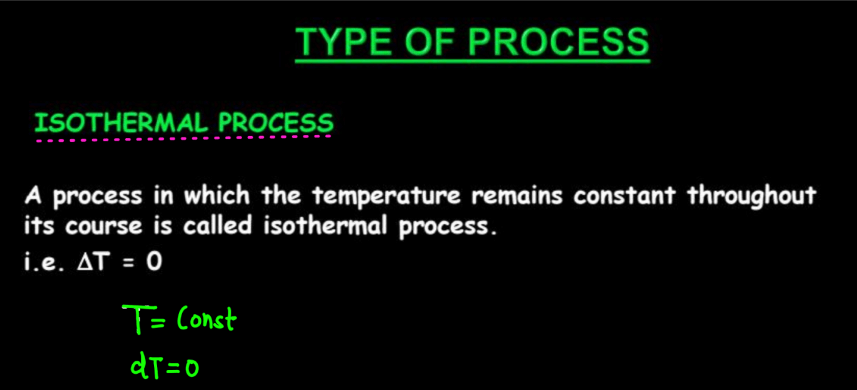
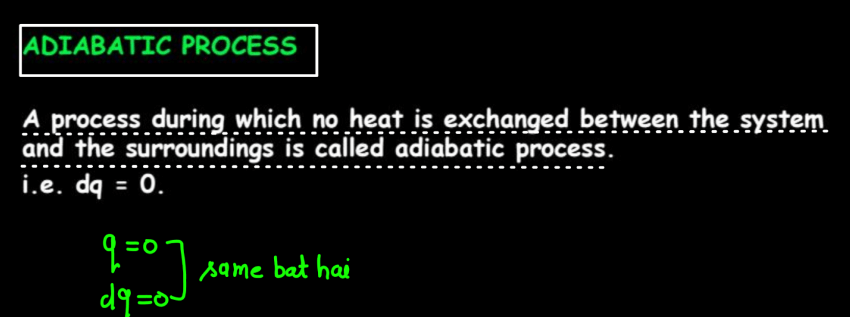
There are four main types of thermodynamic processes namely: Isothermal process, Adiabatic process, Isobaric process, and Isochhoric process. Each type of thermodynamic process represents a specific set of conditions and behavior within a thermodynamic system.
| Different Thermodynamic Processes | |
| Types | Description |
| Isothermal Process | The isothermic processes occur at constant temperatures. |
| Adiabatic Process | No heat exchange with the surroundings occurs in the adiabatic process. |
| Isobaric Process | The isobaric process takes place at constant pressure. |
| Isochoric Process | The isochoric process is also known as an isovolumetric or constant volume process. |


Thermodynamic Equilibrium
There are many different types of thermodynamic equilibrium namely: Mechanical Equilibrium, Thermal Equilibrium, Chemical Equilibrium, Phase Equilibrium, and Radiative Equilibrium. Achieving these equilibria is crucial to understanding and analyzing thermodynamic systems.
| Different Types of Thermodynamic Equilibrium | |
| Types | Description |
| Mechanical Equilibrium | No net force acts within a system, meaning there is no overall movement of the system. |
| Thermal Equilibrium | Uniform temperature throughout the system, indicating no heat transfer between different parts. |
| Chemical Equilibrium | The rates of forward and reserve chemical reactions are equal, resulting in a constant concentration of reactants and products. |
| Phase Equilibrium | The coexistence of different phases (solid, liquid, gas) with stable properties, and no net mass transfer between phases. |
| Radiative Equilibrium | The balance between upcoming and outgoing radiation is often applied to celestial bodies like stars. |
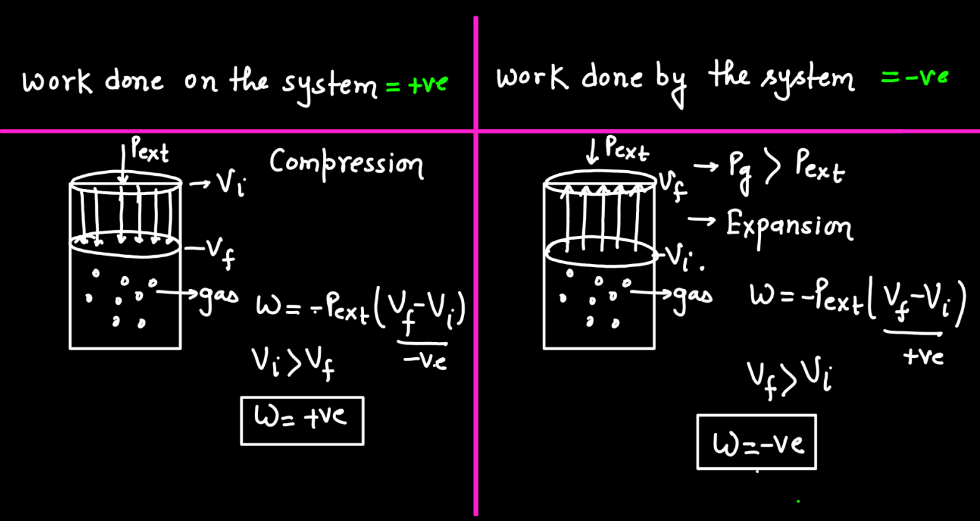
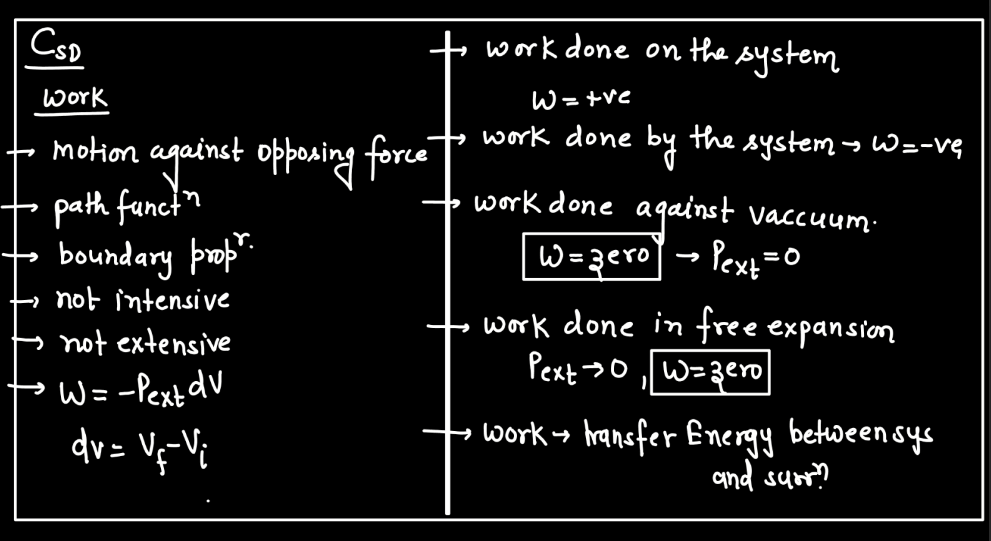
Work
In thermodynamics, work is the transfer of energy between a system and its surroundings that results in a change in the system’s state. This energy transfer can occur through mechanical means, such as compression or expansion of gases, or through processes like electrical work. Work is a crucial concept in thermodynamics because it helps qualify the changes in a system’s internal energy. When a system performs work, it either gains or loses energy, influencing its temperature, pressure, and volume. Work is expressed mathematically as the product of force and displacement in mechanical work, or in various forms depending on the type of energy transfer. Understanding work is fundamental to analyzing and predicting the behavior of thermodynamic systems.

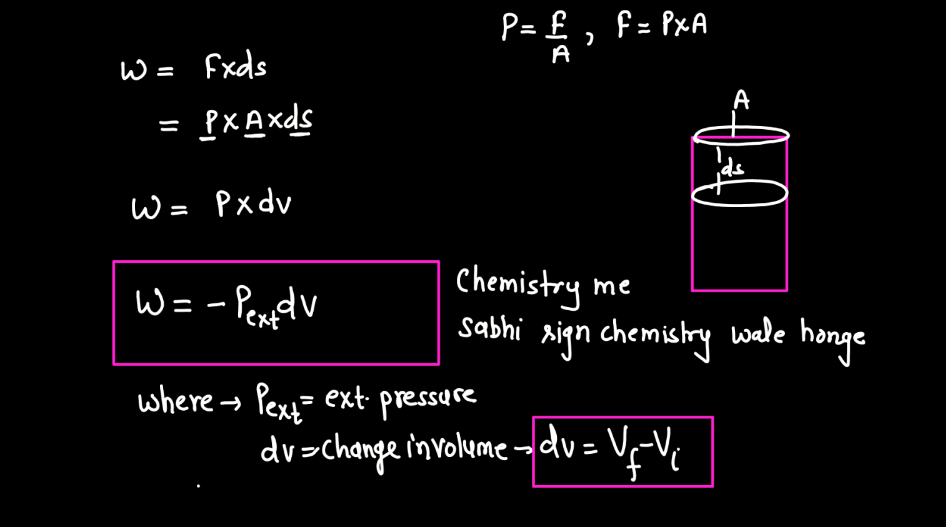
Heat
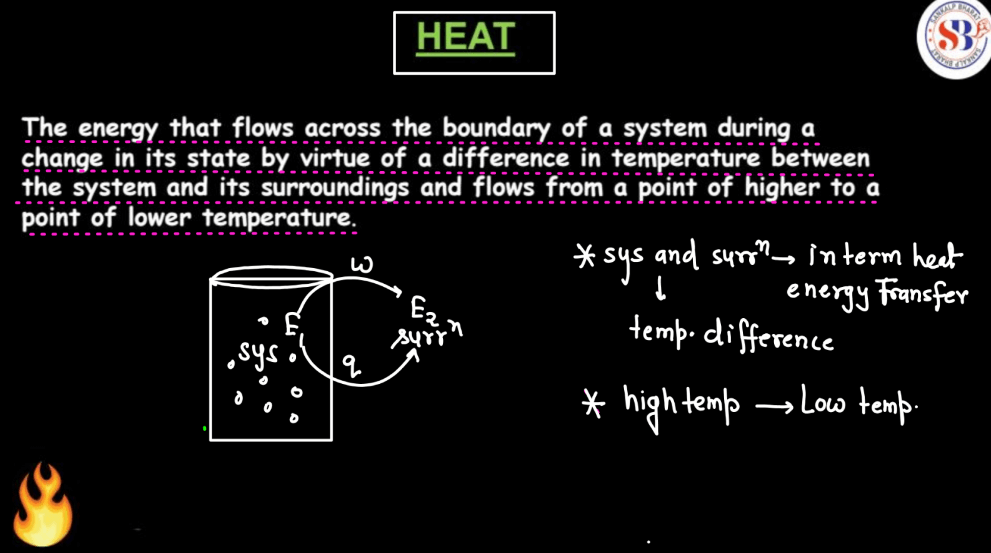
Heat in thermodynamics refers to the transfer of energy between a system and its surroundings due to a temperature difference. It is a form of energy that flows from a region of higher temperature to a region of lower temperature. Unlike work, which involves mechanical processes, heat transfer can occur through conduction, convection, or radiation. Conduction is the transfer of heat through direct contact, convection involves the movement of fluids (liquids or gases), and radiation is the emission of electromagnetic waves. Heat is crucial in understanding how energy is exchanged within a system, impacting its temperature and influencing various thermodynamic properties. It plays a central role in defining the behavior of substances and the overall dynamics of thermodynamic processes.
For Detailed Study, Watch the video below:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













