What is a Valence Bond Theory?
Valence Bond Theory (VBT) is a model in chemistry that explains how chemical bonds form between atoms. It suggests that when atoms approach each other, their atomic orbitals (regions where electrons are likely to be found) overlap. As a result, the electrons in these overlapping orbitals create a shared region, forming a covalent bond. The Valence Bond theory emphasizes the importance of electron pairing in these overlaps, representing the shared electron pair as a bond.
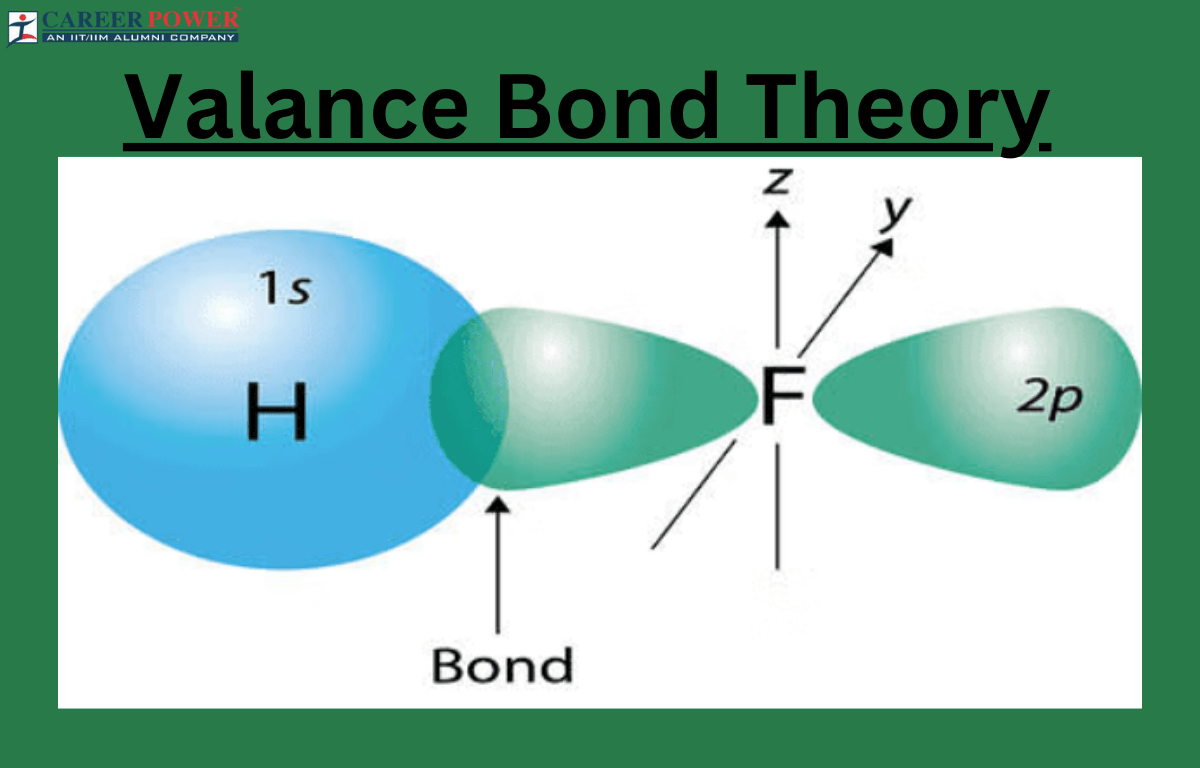
Additionally, VBT accounts for the directional nature of bonds, explaining why molecules have specific shapes. For example, in a hydrogen molecule, two hydrogen atoms come together, and their 1s atomic orbitals overlap, forming a sigma bond. Valence Bond Theory provides a visual and intuitive understanding of bonding, though it has some limitations compared to other models, like Molecular Orbital Theory.
Discovery of Valence Bond Theory
In the 20th century, Linus Pauling proposed the Valence Bond Theory to explain how atoms form chemical bonds. He suggested that when atoms approach each other, their orbitals (regions where electrons are likely to be found) can overlap, leading to the formation of covalent bonds. In this theory, electrons are shared between atoms, and the stability of the resulting molecule is attributed to the overlapping orbitals. Pauling’s model helps us understand the shapes and properties of molecules by considering the specific orbitals involved in bonding. Valence Bond Theory provides a visual framework for comprehending chemical bonding, aiding scientists in predicting molecular structures and behaviors. It significantly contributed to our understanding of molecular chemical chemistry and remains a fundamental concept in the field.
Hybridization
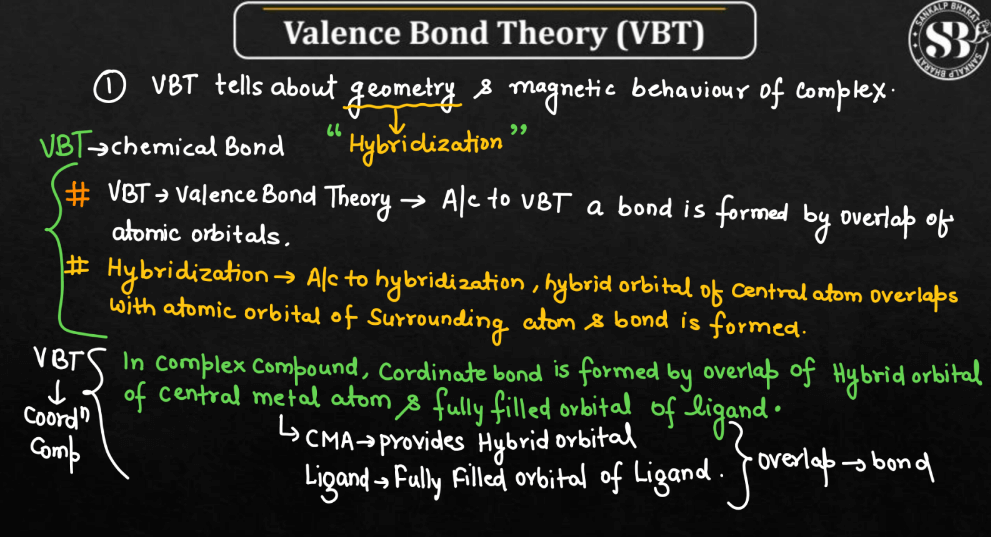
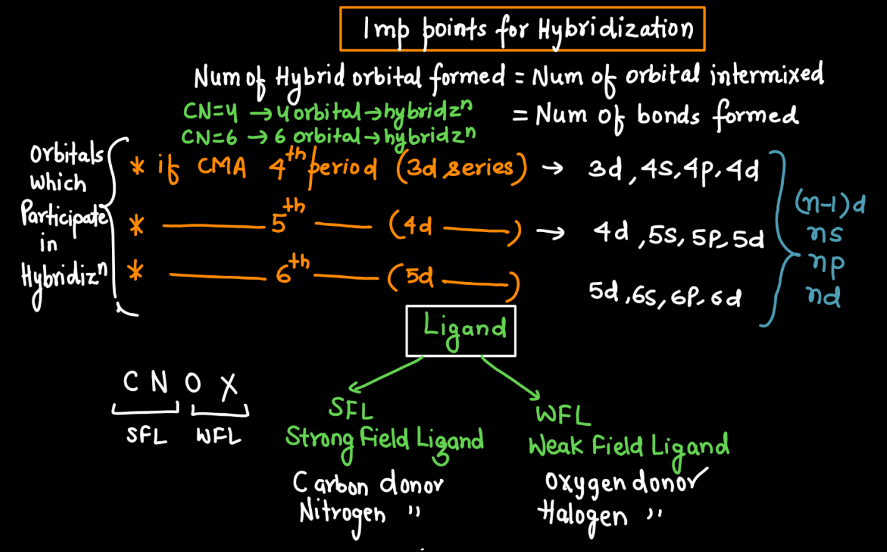
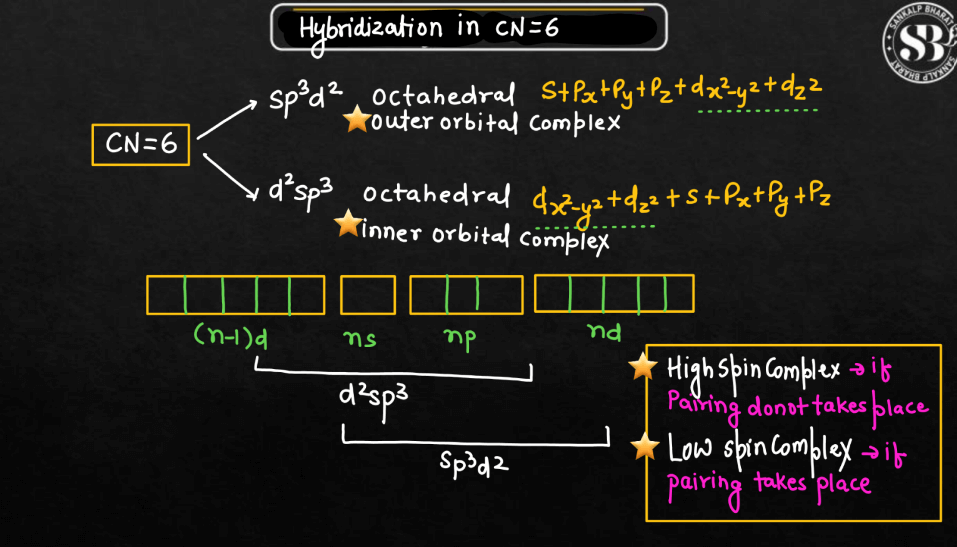
In Vlance Bond Theory, hybridization is a concept that explains the formation of molecular orbitals in covalent compounds. When atoms bond, their atomic orbitals with different shapes and energy levels. For example, when carbon forms four bonds in methane, it undergoes sp3 hybridization, combining one 2s orbital and three 2p orbitals to create four equivalent sp3 hybrid orbitals. These hybrid orbitals then overlap with the orbitals of other atoms to form strong sigma bonds. Hybridization allows us to understand the molecular geometry and predict the shapes of molecules. It simplifies the complex nature of atomic orbitals, providing a practical way to describe the bonding in molecules using a set of hybrid orbitals tailored for specific arrangements.
Some Facts About Valence Bond Theory
Valence Bond Theory (VBT) is a model used in chemistry to explain chemical bonding. Remember, while Valency Bond Theory is a valuable tool, it’s part of a broader understanding of chemical bonding that also includes Molecular Orbital Theory and other models. Here are a few interesting facts about it:
- Resonance: VBT accommodates resonance, a phenomenon where multiple Lewis structures can be drawn for a molecule. It suggests that the actual structure is a resonance hybrid of the contributing structures.
- Application in Coordination Compounds: VBT is commonly used to explain the bonding in coordination compounds, particularly the formation of metal-ligand bonds.
- Geometry Predictions: VBT provides a qualitative understanding of molecular geometry based on the type and number of hybrid orbitals formed. It can predict bond angles and shapes in a straightforward manner for many simple molecules.
- Historical Development: Valence Bond Theory (VBT) was developed by Linus Pauling in the 1930s. Pauling, a renowned American chemist, received the Nobel Prize in chemistry in 1954 for his contributions to the understanding of the nature of the chemical bond.
- Localized Electron Paris: VBT focuses on localized electron pairs and the overlap of atomic orbitals directly involved in bonding. This makes it particularly useful for explaining the bonding in simple molecules.
- Spin Pairing: VBT addresses the issue of spin pairing in the formation of covalent bonds. Electrons with opposite spins occupy the overlapping orbitals to achieve a more stable configuration.
Postulates of Valence Bond Theory
Valence Bond Theory (VBT) is a model in Chemistry that explains the formation of chemical bonds. Remember, while VBT provides valuable insights, it has limitations and is often complemented by other theories like Molecular Orbital Theory for a more comprehensive understanding of molecular structure and bonding. Here are the widely explained postulates:
- Orbital Overlap: Valence Bond Theory emphasizes the overlap of atomic orbitals to form covalent bonds. The greater the overlap, the stronger the bond.
- Directionality of Bonds: VBT suggests that bonds have a directional nature and the overlap of orbitals occurs along specific axes. This explains the shape and geometry of molecules.
- Hybridization: Valence Bond Theory introduced the concept of hybridization to explain molecular shapes that couldn’t be easily explained by individual atomic orbitals. It involves the mixing of atomic orbitals to form hybrid orbitals.
- Sigma and Pi Bonds: VBT distinguishes between sigma bonds (σ), formed by the head-on overlap of orbitals, and pi bonds (π), formed by the side-to-side overlap of parallel orbitals. This helps explain the characteristics of double and triple bonds.
- Magnetic Properties: The theory accounts for the magnetic properties of molecules by considering the alignment or misalignment of magnetic moments resulting from unpaired electrons in overlapping orbitals.
Limitations of Valence Bond Theory
While Valence bond theory is a useful concept for understanding the basis of the concept of Valence Bond Theory for understanding basics of chemical bonding, Molecular Orbital Theory provides a more comprehensive explanation for a wide range of molecular properties.
- Overlapping Considerations: It mainly focuses on the overlap of atomic orbitals to form bonds, neglecting other factors like resonance and delocalization that are crucial in certain molecules.
- Inability to explain Molecular Shapes: Valence Bond Theory struggles to explain molecular shapes. Molecular orbital Theory is often more effective in describing molecular geometry.
- Limited Scope for Complex Molecules: It becomes increasingly challenging to apply Valence bond theory to complex molecules where multiple resonance structures or delocalized electrons play a significant role.
- Assumption of Localized Electrons: Valence Bond Theory assumes that electrons are localized between two atoms, which may not be accurate for molecules with extensive delocalization.
- Paramagnetic Behavior Prediction: It may incorrectly predict paramagnetic behavior in certain molecules due to its localized electron model.
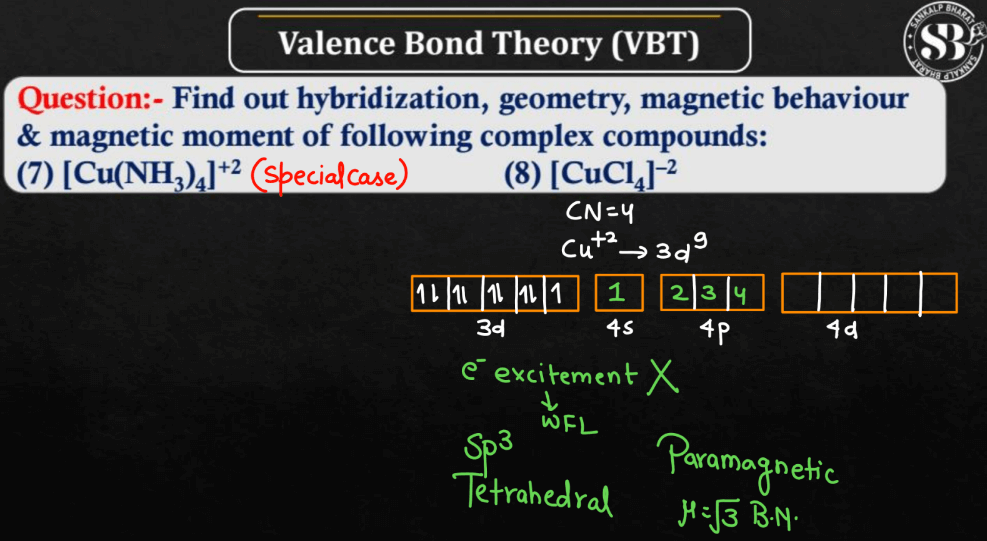
MCQs on Valence Bond Theory (VBT)
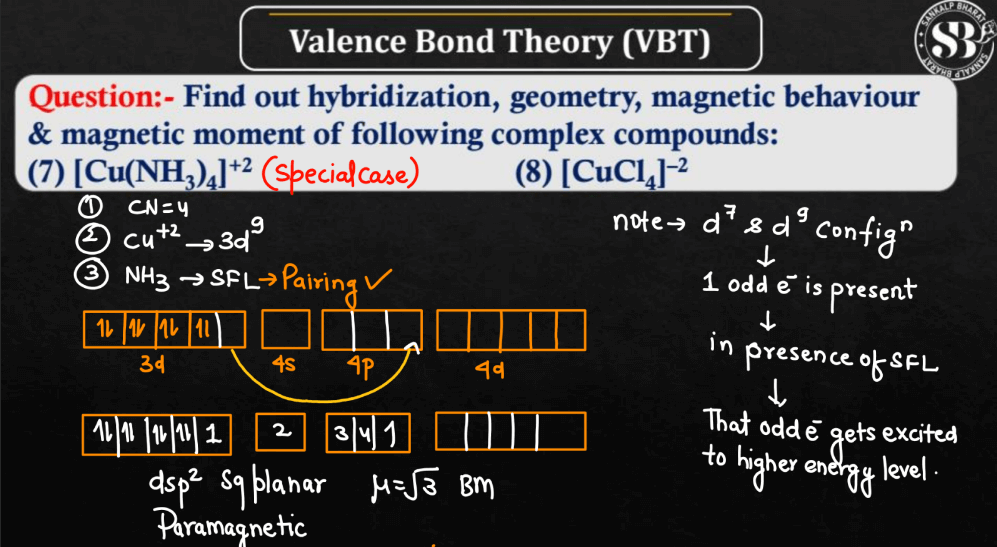
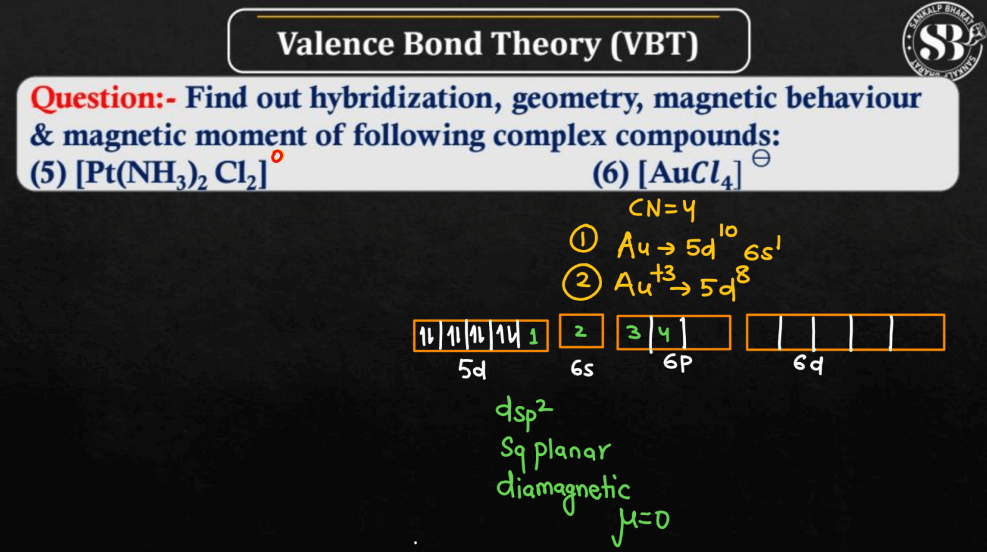
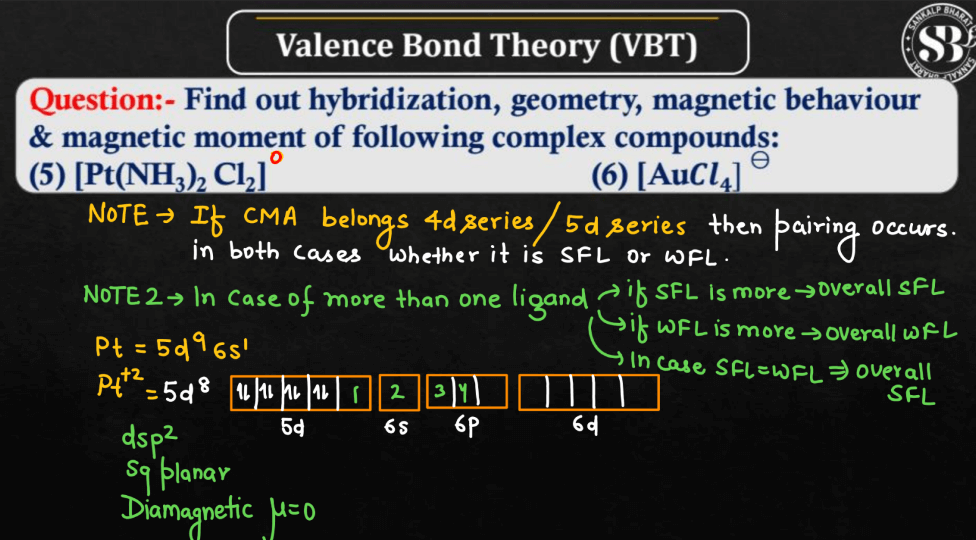
For More Details, Watch the below video:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













