First Law of Thermodynamics
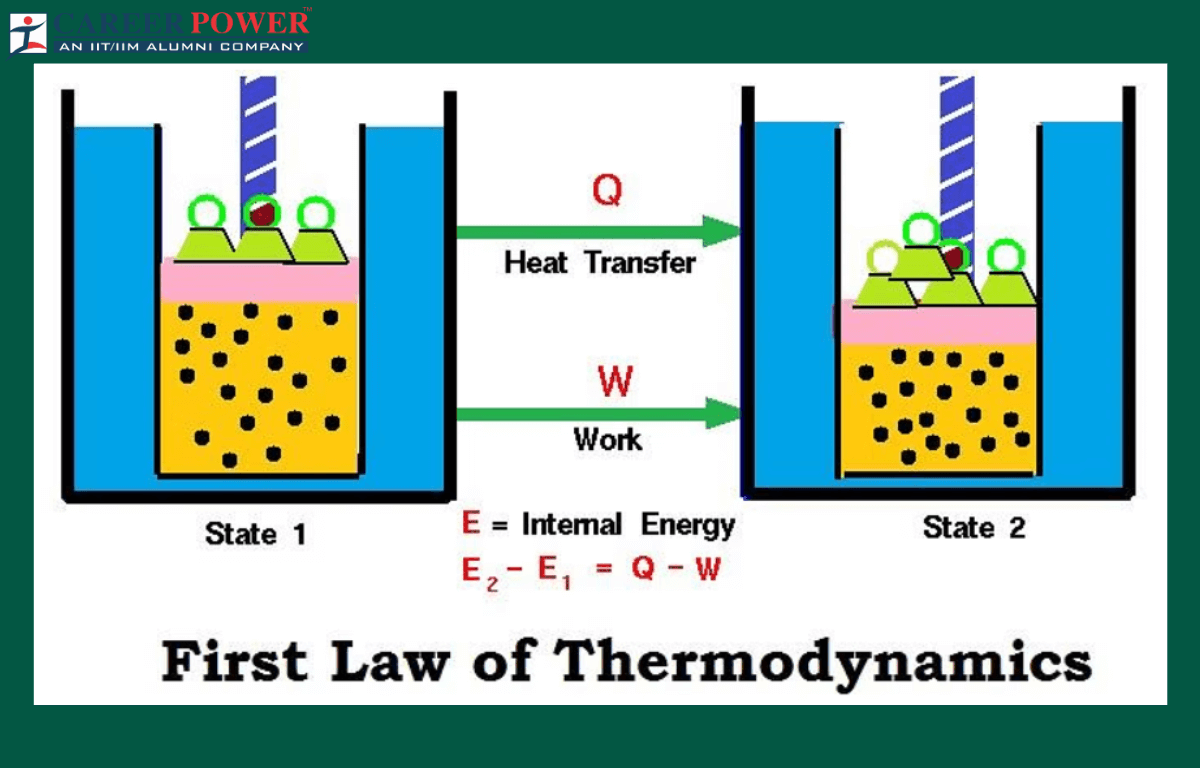
The First Law of Thermodynamics, also known as the law of energy conservation, is a fundamental principle governing the behavior of energy in physical systems. In straightforward terms, it asserts that the total energy with a closed system remains constant; it can not be created or destroyed, only transformed from one form to another. Picture a sealed container filled with gas: when heat is added, the internal energy rises as the gas molecules gain kinetic energy, causing the pressure to increase. Conversely, removing heat reduces the internal energy, slowing down the molecules.
This law encapsulates the idea that energy is a persistent entity, undergoing various conversions between potential, kinetic, and other forms. Whether it’s a moving piston, a chemical reaction, or a flowing river, the total energy of the system remains unchanged. The first law of thermodynamics is a cornerstone in understanding the principles governing energy interactions, providing a foundation for studying heat, work, and the dynamic transformations occurring in diverse physical processes.

Derivation of First Law of Thermodynamics
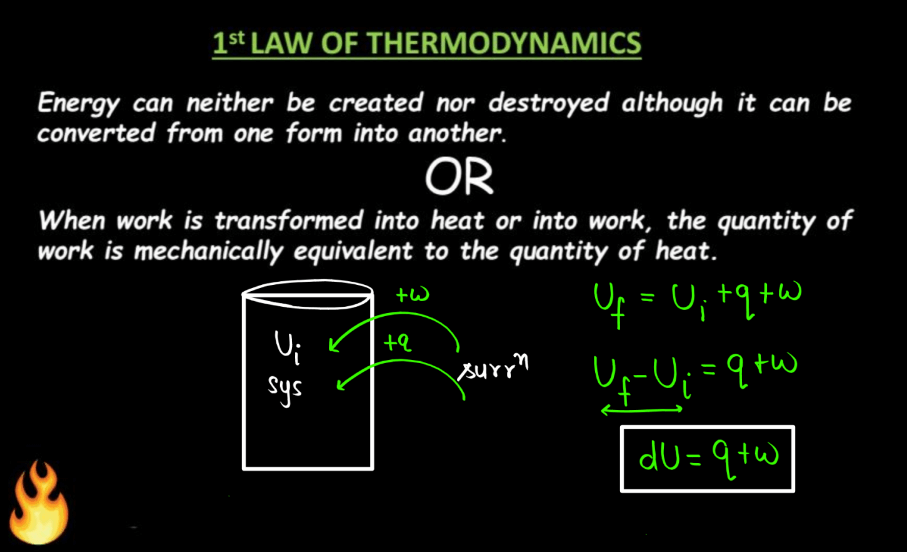
The 1st Law of Thermodynamics states that energy can neither be created nor destroyed, only transferred or converted from one form to another. The derivation often starts with the concept of internal energy (U), which includes both the kinetic and potential energy of particles within a system. The equation can be expressed as:
ΔU = Q + W
Where ΔU is the change in internal energy of the system, Q is the Algebraic sum of heat transfer between the system and surroundings, and W is the work done by the system on its surroundings or vice versa.
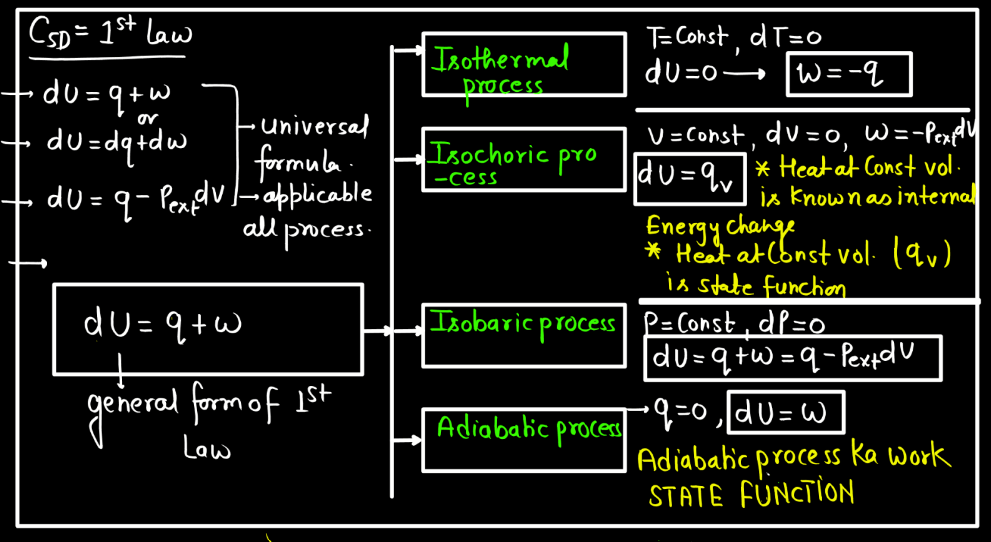
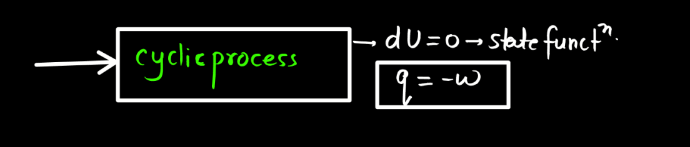
For a closed system undergoing a thermodynamic process, the first law is based on the principles of conservation of mass and energy, considering the work done on the system and the heat exchanged with the surroundings. Different derivations may involve specific scenarios, such as constant volume or constant pressure processes, and may include expressions like PV work, but the essence remains the same: the change in internal energy equals the heat added minus the work done.
Some Facts About 1st Law of Thermodynamics
Some of the Interesting facts about the first law of thermodynamics have been discussed below:
- Conservation of Energy: The first law of thermodynamics asserts that the total energy in an isolated system is constant; it can neither be created nor destroyed.
- Energy Transformation: While the total energy remains constant, it can undergo various transformations, such as changing from potential to kinetic energy or converting between heat and work.
- Internal Energy Changes: The law encompasses internal energy changes within a system, including the heat added or removed and the work done on or by the system.
- Chemical Reactions: Applied to chemical reactions, the first law helps explain how energy is conserved during processes like combustion or synthesis.
- Fundamental Principle: This law is a fundamental principle in thermodynamics, forming the basis for understanding energy interactions and the limitations on what can be achieved in energy conservation.
Limitations of First Law of Thermodynamics
The limitations of the first law of thermodynamics have been discussed here:
- Direction of Processes: The first law doesn’t specify the direction in which processes occur. While it tells us energy is conserved, it doesn’t explain why some processes happen spontaneously while others require external influences.
- Efficiency of Conversions: It doesn’t provide information on the efficiency of energy conversions. Some processes may be less efficient due to factors like heat loss, but the first law doesn’t quantify this.
- Qualitative Aspects: The law doesn’t delve into the qualitative aspects of energy transformations. For example, it doesn’t explain the irreversibility of certain processes.
- Spontaneity and Entropy: The concept of entropy, a measure of disorder or randomness in a system, is not explicitly addressed. Understanding the spontaneity of processes requires considering entropy changes, which the first law alone doesn’t cover.
For More Information, Do watch the video given below:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













