What is Nuclear Force?
Nuclear forces are fundamental interactions that bind protons and neutrons within an atomic nucleus. The discovery of nuclear forces is attributed to Hideki Yukawa, a Japanese physicist. In 1935, Yukawa proposed the existence of a new particle called the meson as the carrier of the strong force that binds protons and neutrons together in the atomic nucleus. There are two main types of nuclear forces: the strong nuclear force and the weak nuclear force. Imagine the nucleus as a tight-knit community where protons and neutrons live. Despite the natural tendency for positively charged protons to repel each other due to electromagnetic forces, the nuclear forces act like invisible glue, holding the community together.
These forces operate over very short distances, acting within the confines of the nucleus. They are incredibly strong, overpowering the electromagnetic forces that would otherwise push protons apart. Neutrons play a crucial role in this dynamic by helping to mediate the interactions between positively charged protons. The delicate balance of nuclear forces allows atoms to form, creating the foundation of matter. Understanding these forces is fundamental to comprehending the structure and behavior of atoms, paving the way for advancement in nuclear physics and technology.
Discovery of Nuclear Forces
The discovery of nuclear forces revolutionized our understanding of matter and energy. In the early 20th century, scientists like Ernest Rutherford conducted experiments that revealed the existence of a tiny, dense nucleus at the center of atoms. Further investigation led to the realization that powerful forces, later termed nuclear forces, were at play within the nucleus. These forces, distinct from electromagnetic forces, are responsible for binding protons and neutrons together. The breakthrough came with the development of quantum mechanics, a branch of physics that provided a theoretical framework for understanding these forces. The discovery of nuclear forces not only laid the foundation for nuclear physics but also played a very important role in the development of nuclear technologies, including nuclear power and weapons.
Types of Nuclear Forces
There are two main types of nuclear forces namely: Weak Nuclear Forces and Strong Nuclear Forces. Below we have defined both types of Nuclear forces.
| Types of Nuclear Forces | |
| Types | Description |
| Weak Nuclear Forces | The weak nuclear forces are responsible for processes like beta decay in radioactive nuclei, involving the transformation of one type of elementary particle into another. |
| Strong nuclear Forces | The strong nuclear forces are the most powerful of the four, binding protons and neutrons within atomic nuclei. It acts over extremely short distances, holding the nucleus together despite the repulsive electromagnetic forces between positively charged protons. |
Properties of Nuclear Forces
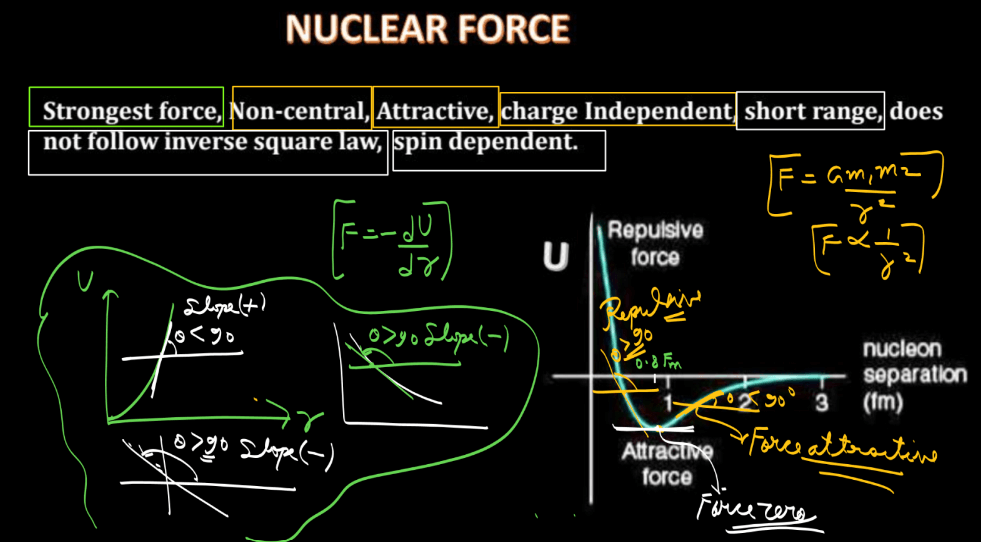
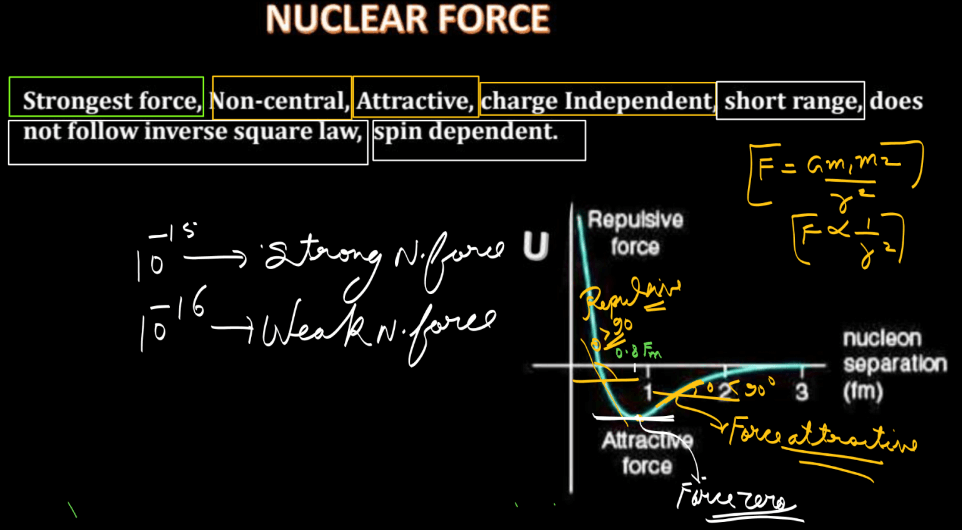
Nuclear forces are short-range forces that act between nucleons (protons and neutrons) within an atomic nucleus. Here we have discussed a few points that highlight the properties of nuclear forces. Understanding these properties is crucial for explaining the stability and structure of atomic nuclei.
- Short Range: Nuclear forces operate within the nucleus and have a very short range, typically less than 1 femtometer.
- Strong Attraction: Nuclear forces are attractive and overcome the electrostatic repulsion between positively charged protons, holding the nucleus together.
- Saturation: Nuclear forces are saturating, meaning they become stronger as nucleons get closer together, preventing the nucleus from collapsing due to the electrostatic repulsion.
- Charge Independence: Nuclear forces are nearly independent of the charge of the nucleons, acting equally on protons and neutrons. This property is known as charge independence.
- Spin Dependence: Nuclear forces depend on the spin orientation of nucleons. The force is stronger when the spins of interacting nucleons are parallel.
- Non-central Interaction: Nuclear forces are not purely central; they also have tensor and spin-orbit components, contributing to the overall force.


Examples of Nuclear Forces
Some of the examples of Nuclear forces have been discussed below.
- Strong Nuclear Force: The strong nuclear force is one of the fundamental forces in nature that binds protons and neutrons within an atomic nucleus. Short-ranged, acting over distances on the order of femtometers. Strong nuclear forces are much stronger than electromagnetic forces at short distances. Overcomes the electrostatic repulsion between positively charged protons, keeping the nucleus stable.
- Nuclear Binding Energy: The energy required to disassemble a nucleus into its individual protons and neutrons. Arises from the conservation to Einstein’s famous equation E = mc². The nuclear binding energy explains why nuclei are stable and provides the energy released in nuclear reactions.
- Coulombic (Electromagnetic) Repulsion: Arises from the electrostatic repulsion between positively charged protons in the nucleus. Strong nuclear force overcomes this repulsion, preventing the nucleus from disintegrating.
- Shell Model and Pauli Exclusion Principle: The shell model describes the arrangement of nucleons in energy levels or shells within the nucleus. The Pauli Exclusion Principle prohibits identical fermions (such as protons and neutrons) from occupying the same quantum state within a nucleus, influencing the nuclear structure.


 Ohm's Law: Definition, Formula, Limitati...
Ohm's Law: Definition, Formula, Limitati...
 Newton's First Law of Motion: Definition...
Newton's First Law of Motion: Definition...
 Kepler's Laws of Planetary Motion: First...
Kepler's Laws of Planetary Motion: First...













