Atomic Model
The atomic model describes the structure of an atom, the fundamental building block of matter. Proposed by Niels Bohr in 1913, this model envisions electrons orbiting the nucleus in specific energy levels or shells. The nucleus, composed of protons and neutrons, is at the atom’s center, imparting mass. Electrons, with negligible mass, orbit the nucleus in quantized energy states. Subsequent advancements, like the quantum mechanical model, refine our understanding, acknowledging electron probability clouds instead of definite orbitals. Additionally, the Standard Model integrates particles and forces governing atoms. This model is crucial for comprehending chemical properties, bonding, and the diverse elements constituting the universe, playing a foundational role in modern physics and chemistry.
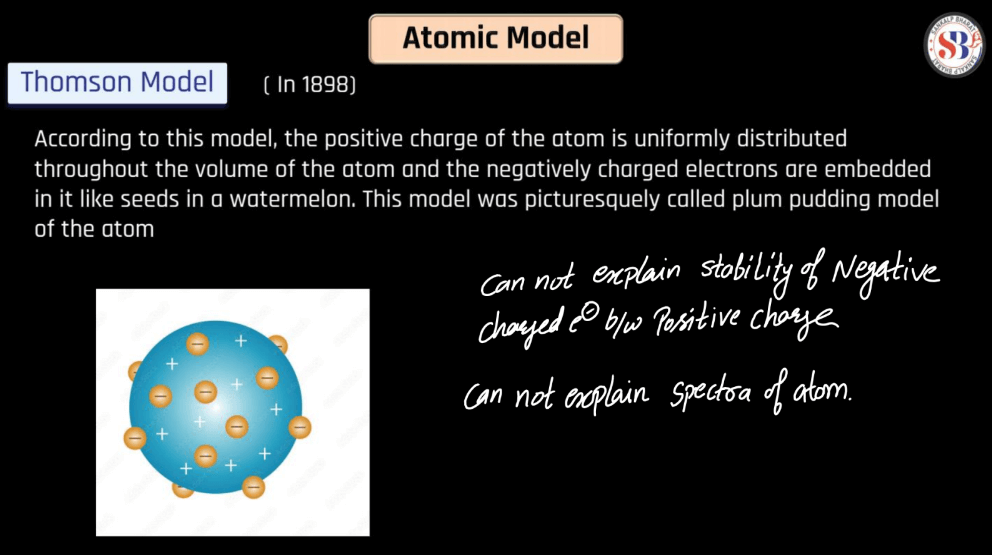
Thomson Atomic Model
The Thomson atomic model, proposed by J.J. Thomson, depicted atoms as indivisible spheres of positive charge with embedded electrons. In this “plum pudding” model, electrons were like raisins distributed within a positively charged pudding. Thomson’s groundbreaking work emerged from his cathode ray experiments, demonstrating that electrons were negatively charged particles. While this model laid the foundation for understanding atomic structure, it was later replaced by the more comprehensive Rutherford Model.
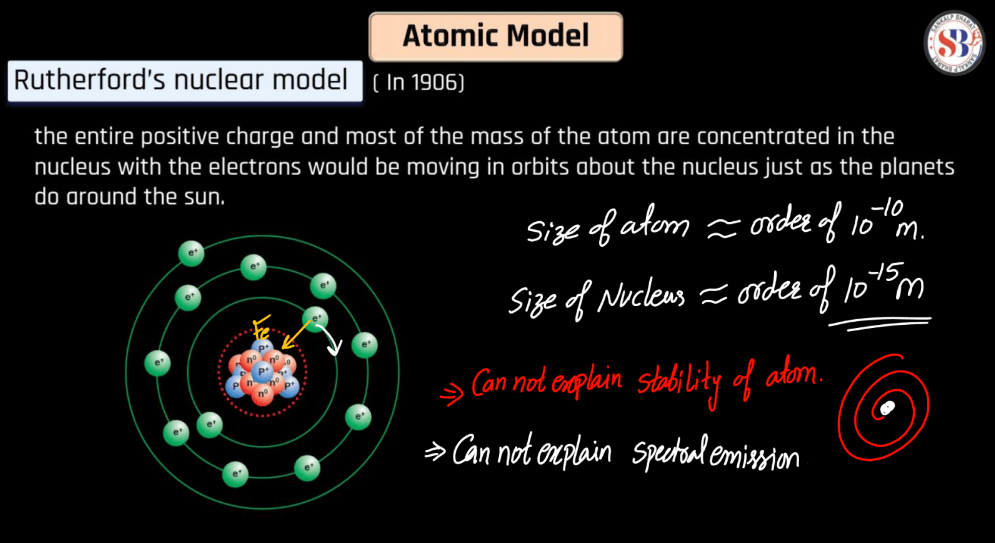
Rutherford Nuclear Model
The Rutherford Nuclear Model proposed by Ernest Rutherford in 1911, revolutionized the understanding of atomic structure. Rutherford’s model suggested that atoms have a small, dense nucleus at their center, containing positively charged protons. Electrons orbit the nucleus at a distance. This model arose from Rutherford’s gold foil experiment, where he observed that most alpha particles passed through the foil, but some were deflected, indicating a concentrated positive charge in the nucleus. The Rutherford model addressed the limitations of the Thomson Model and laid the groundwork for the modern atomic model, eventually evolving into the Bohr model and later quantum mechanical models that provided a more nuanced depiction of atomic behavior.
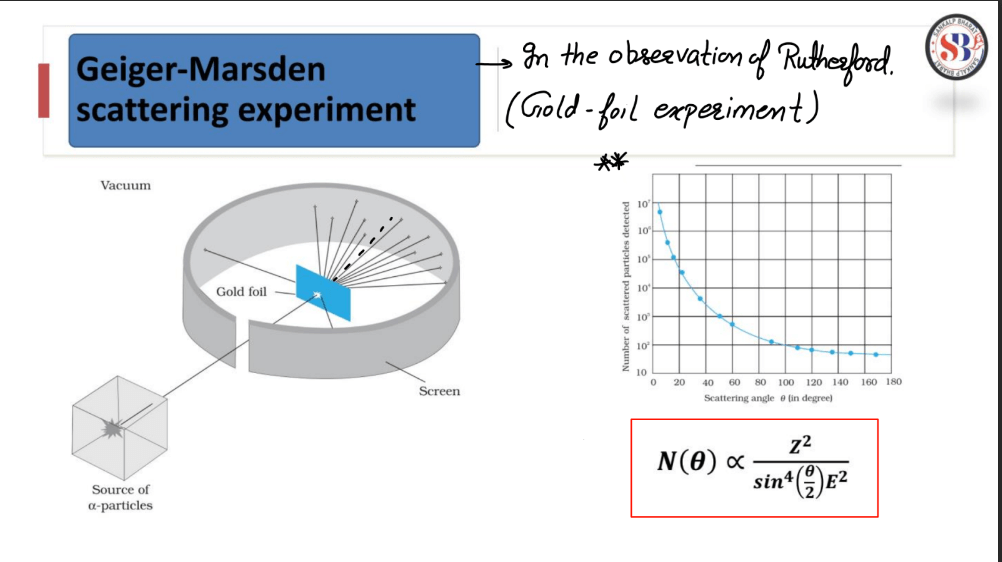

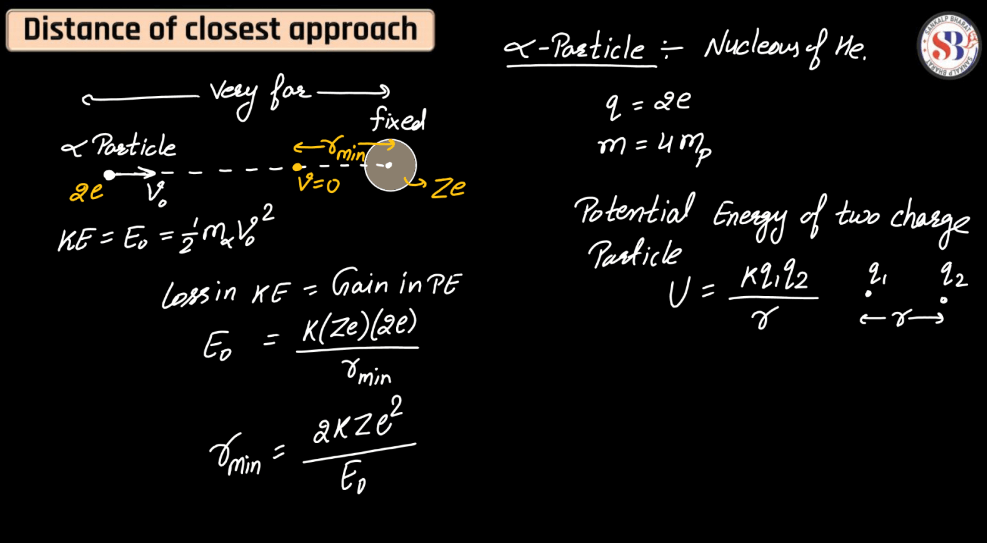
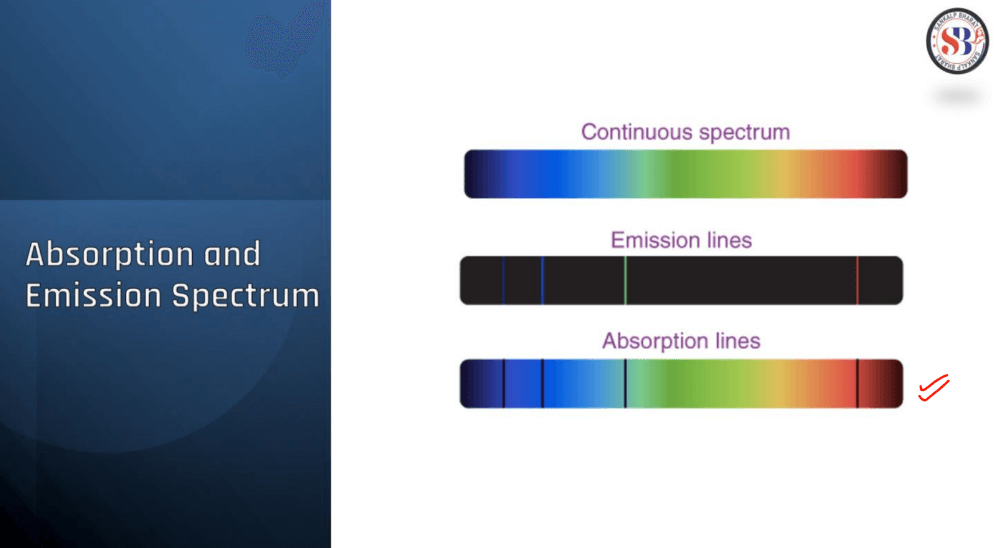
Absorption and Emission Spectrum
Absorption and emission spectra are fundamental concepts in the study of light and matter interactions. An absorption spectrum showcases the wavelengths of light absorbed by a substance, revealing distance dark lines or bands corresponding to specific energy levels. This occurs when atoms or molecules absorb photons, transitioning from lower to higher energy states. Conversely, an emission spectrum displays the wavelengths of light emitted when these excited particles return to lower energy states. Bright lines or bands in the emission spectrum correspond to specific transitions. Together, these spectra provide crucial insights into the composition and behavior of substances, forming the basis for fields such as astronomy, chemistry, and physics.
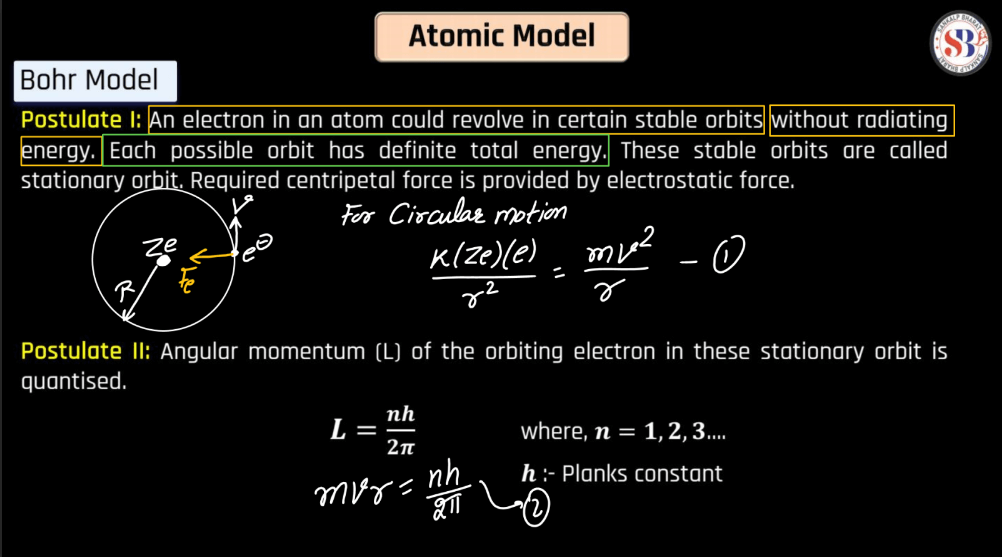
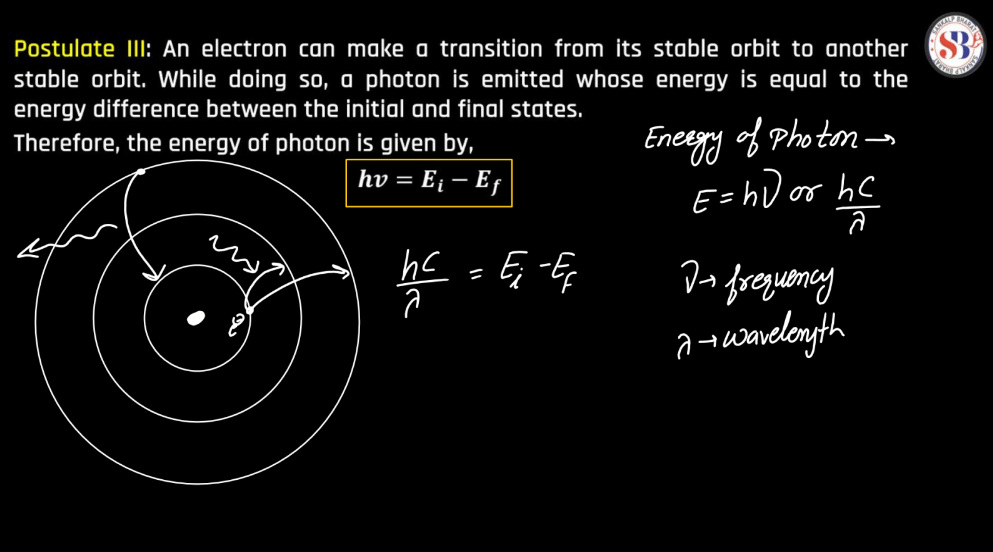
Bohr Atomic Model
The Bohr Atomic Model, proposed by Danish Physicist Niels Bohr in 1913, revolutionized our understanding of atomic structure. Bohr introduced the concept of quantized energy levels, suggesting that electrons orbit the nucleus in discrete orbits, each corresponding to a specific energy level. This model successfully explained the spectral lines of hydrogen and offered a clear explanation for the stability of atoms. According to Bohr, electrons could jump between these orbits by absorbing or emitting discrete amounts of energy, releasing light of specific frequencies. Despite its success in explaining certain phenomena, the Bohr model was later superseded by a more comprehensive quantum mechanical model. Nevertheless, Bohr’s pioneering work laid the foundation for further advancements in atomic theory.
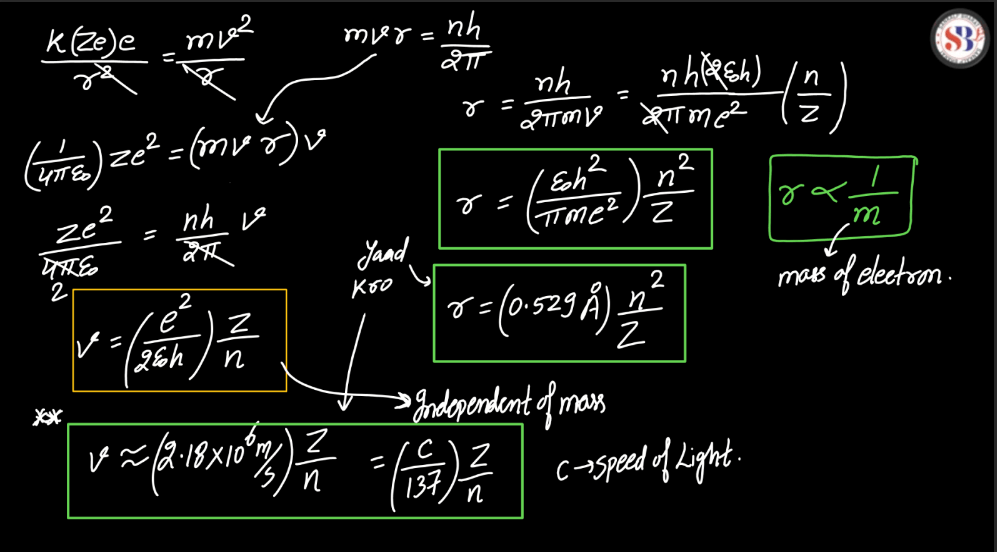
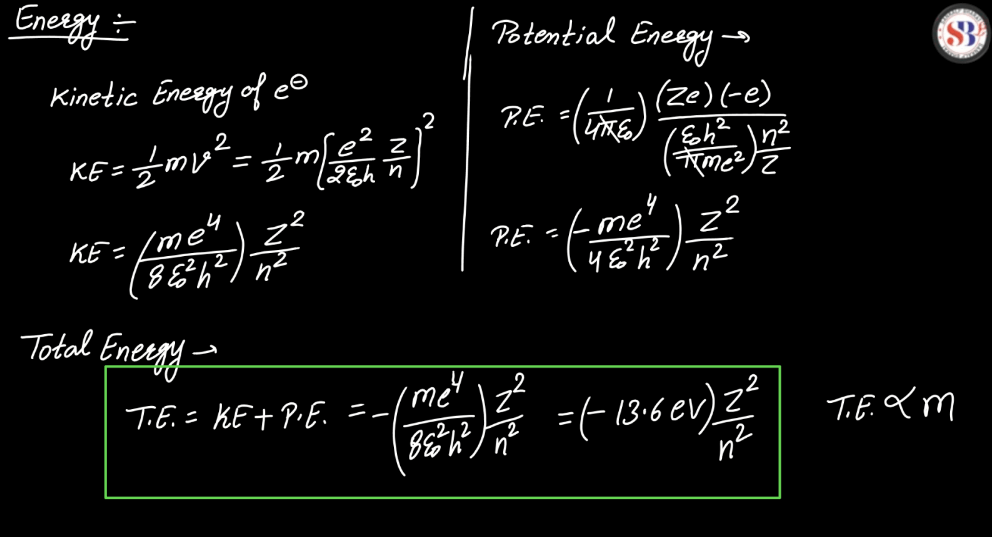

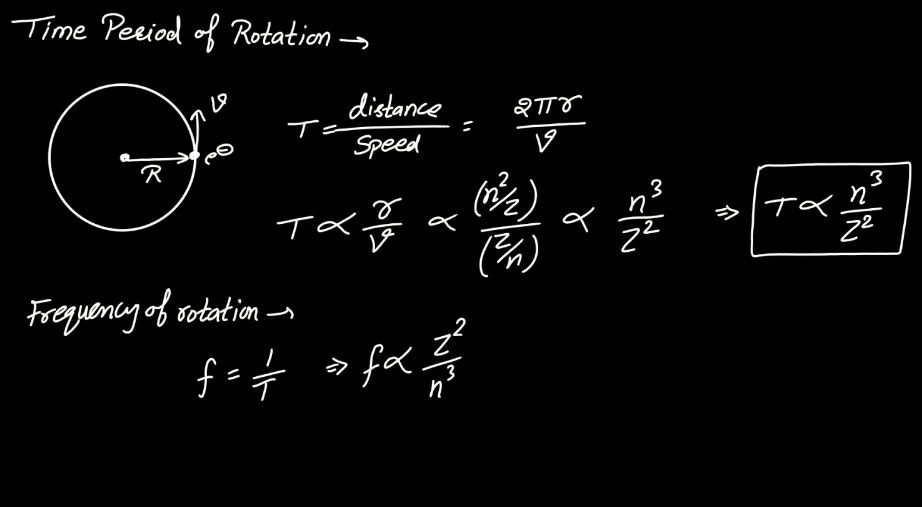
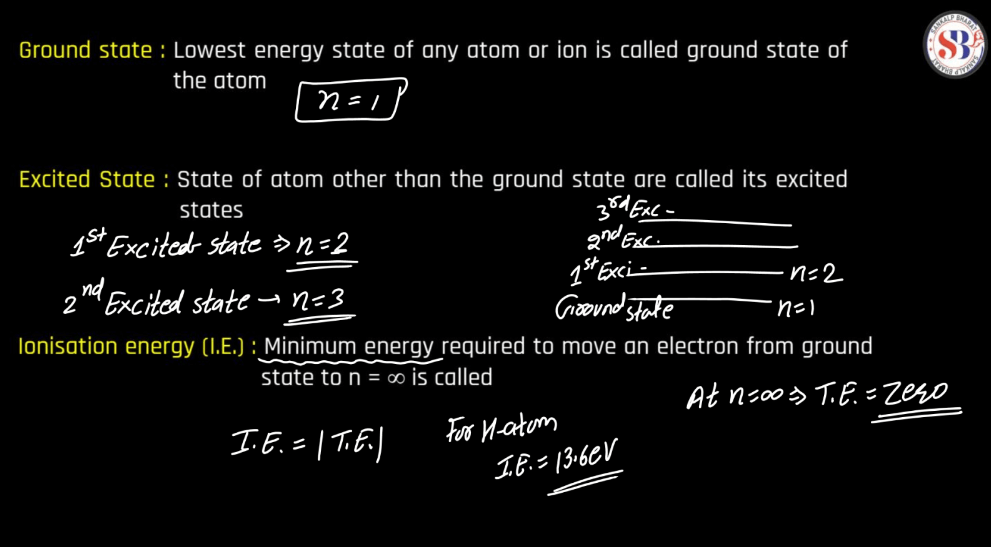
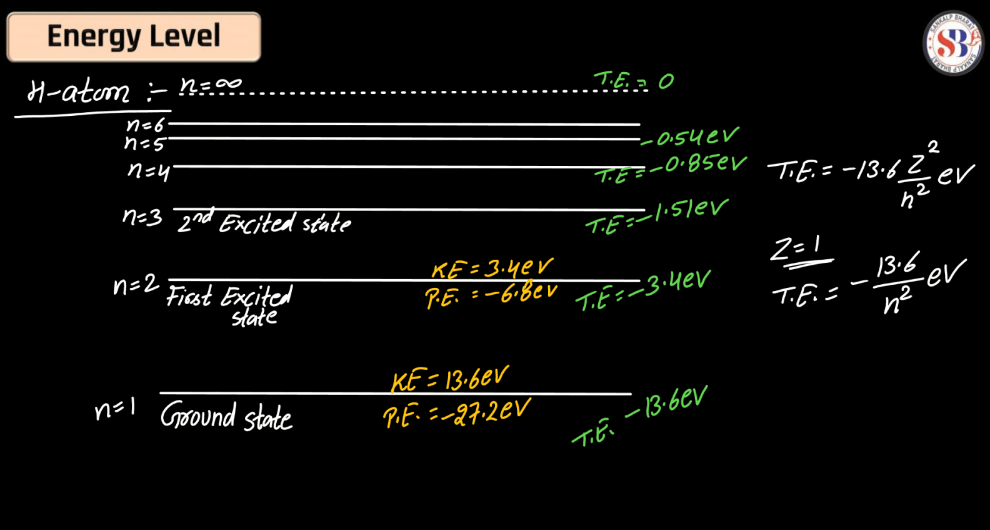
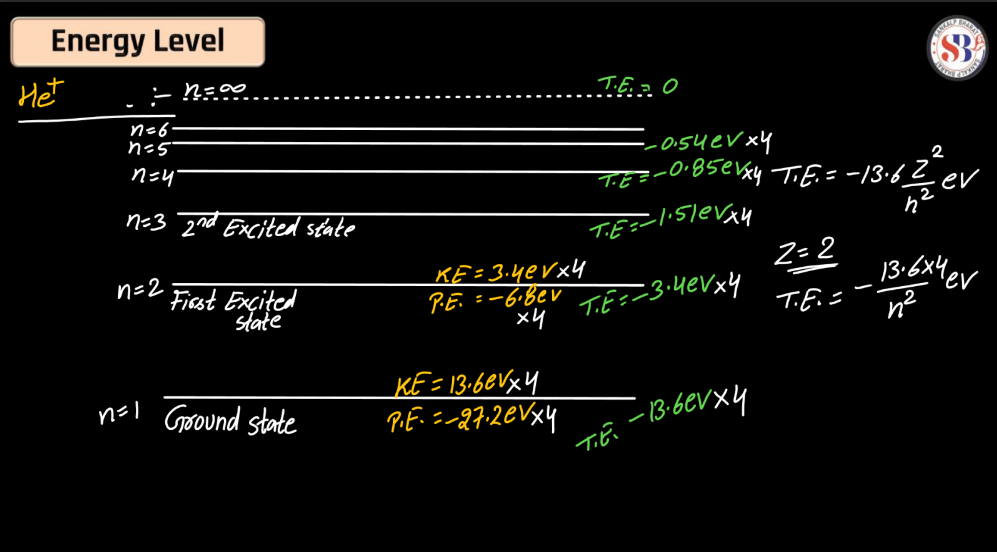
Energy Level
Energy levels refer to the distinct orbits or shells within an atom where electrons orbit the nucleus. These levels are quantized, meaning electrons can only occupy specific energy values. The first level is closest to the nucleus and has the lowest energy, while subsequent levels have higher energies. Electrons can move between these levels by absorbing or emitting energy in the form of photons. This concept plays a pivotal role in comprehending the electronic structure of elements and their participation in chemical reactions.
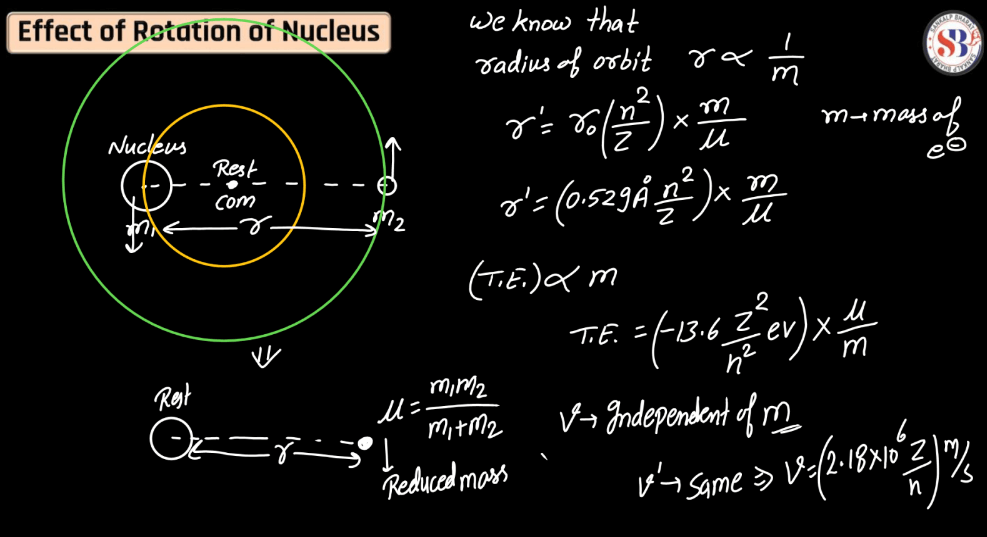
Effect of Rotation of Electrons
The rotation of the nucleus plays a crucial role in nuclear physics, influencing various phenomena. As the nucleus rotates, it induces changes in its angular momentum, affecting the energy levels and stability of the energy levels and stability of the nucleus. This rotation can lead to alterations in nucleus shape, impacting the nuclear binding energy and contributing to the overall behavior of the atom. Additionally, rotational motion influences nuclear magnetic moments, influencing interactions with external magnetic fields. Understanding the effects of nucleus rotation is essential for comprehending nuclear structure, stability, and reactions, contributing significantly to both theoretical nuclear physics and practical applications in fields such as nuclear energy and medicine.
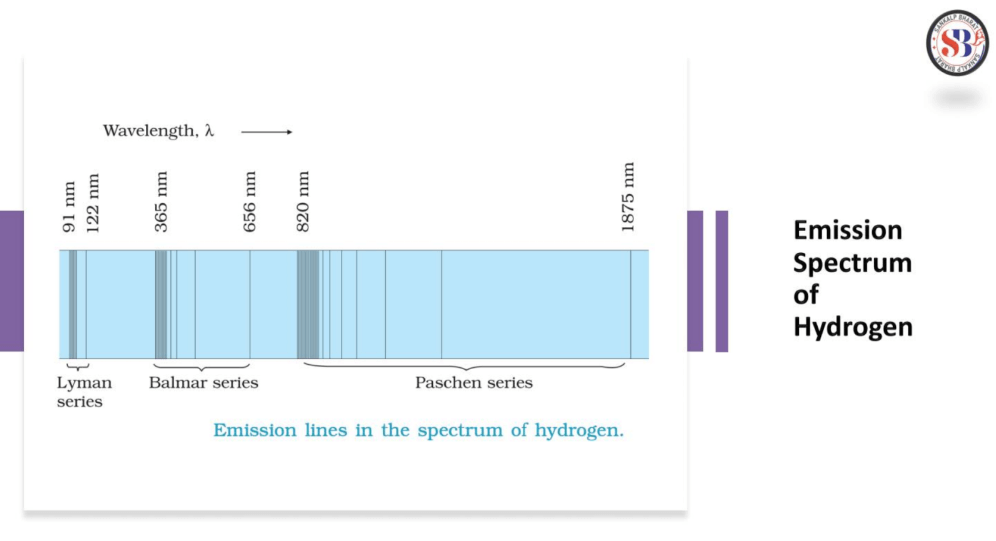
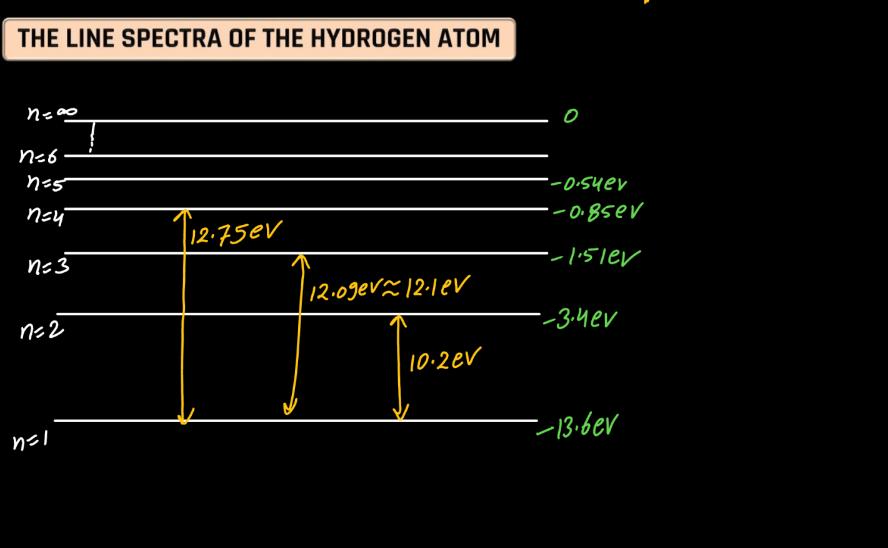
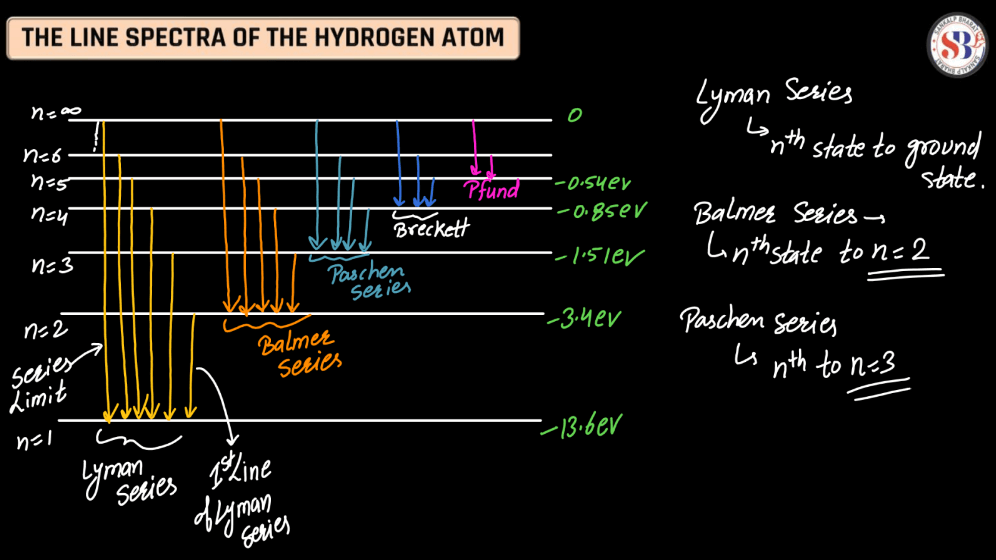
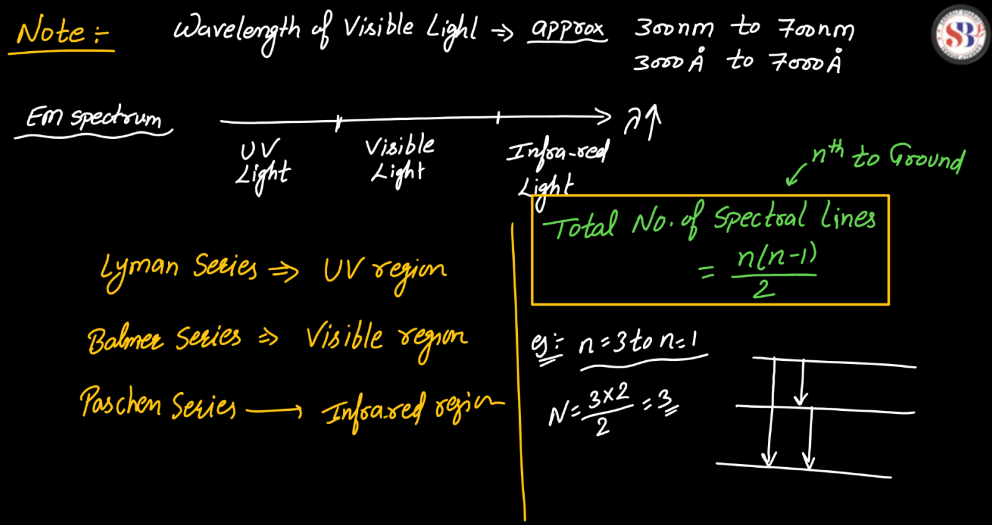
Line Spectra of Hydrogen Atom
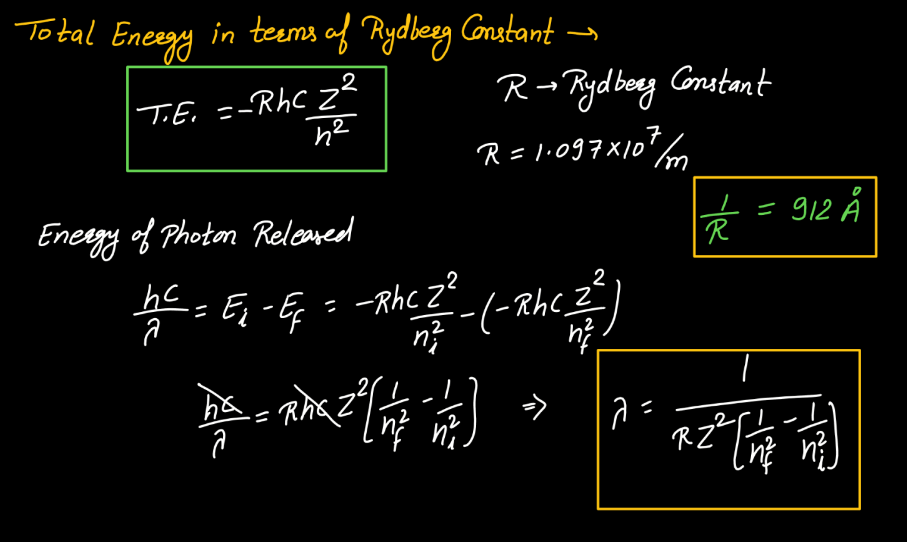
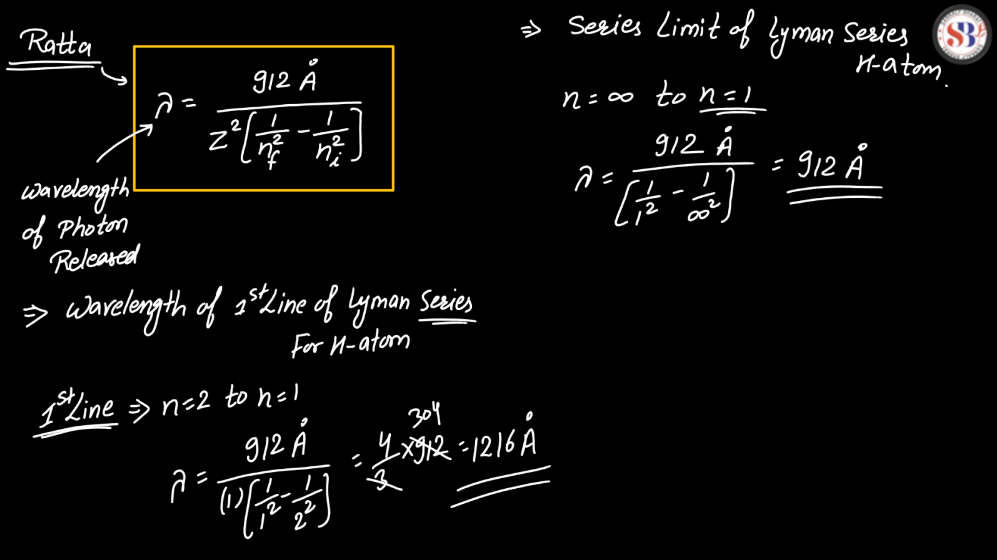
The line spectra of a hydrogen atom result from the quantized energy levels of its electrons. When an electron transitions between these energy levels, it emits or absorbs energy in the form of light, creating distinct lines in the spectrum. Each line corresponds to a specific energy change, and the wavelength of these lines can be explained by the Balmer, Lyman, and Paschen series, representing different electron transitions. The simplicity and precision of hydrogen’s line spectra played a crucial role in the development of quantum theory, providing evidence for quantized energy levels in atoms.
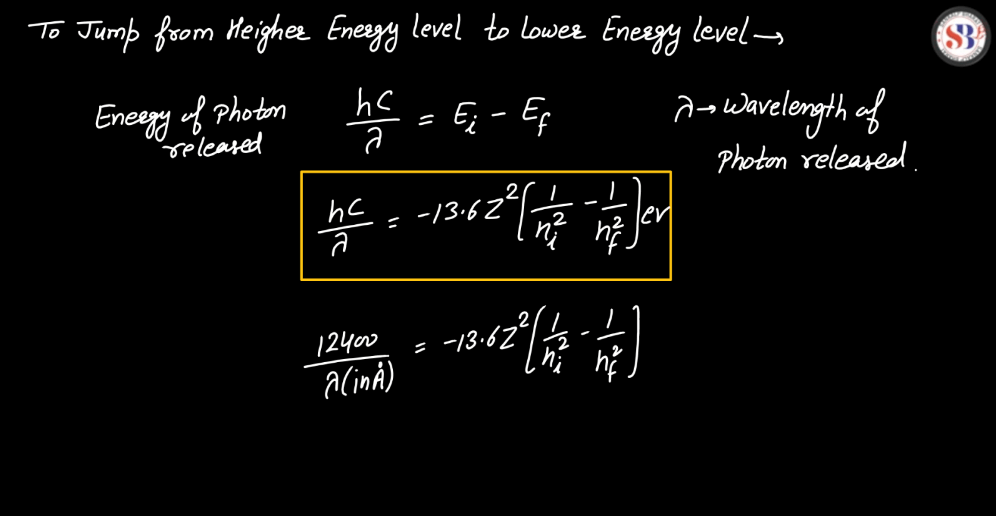
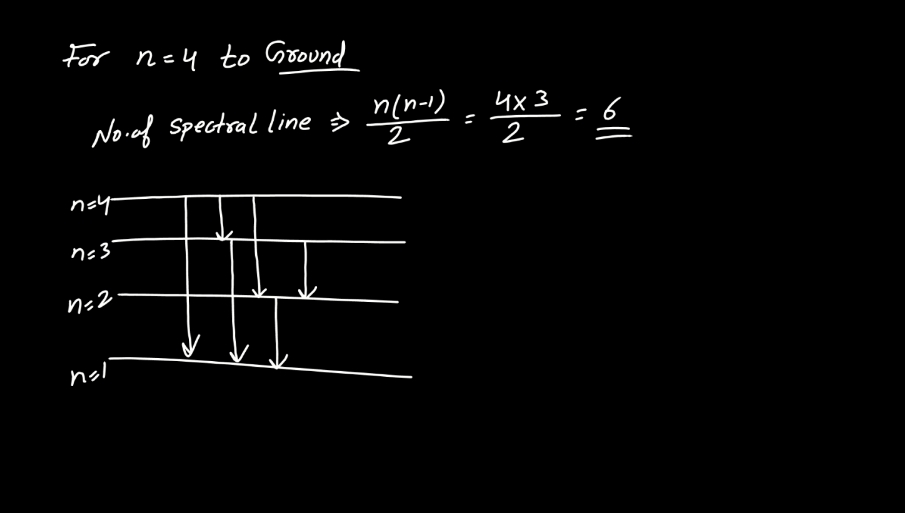
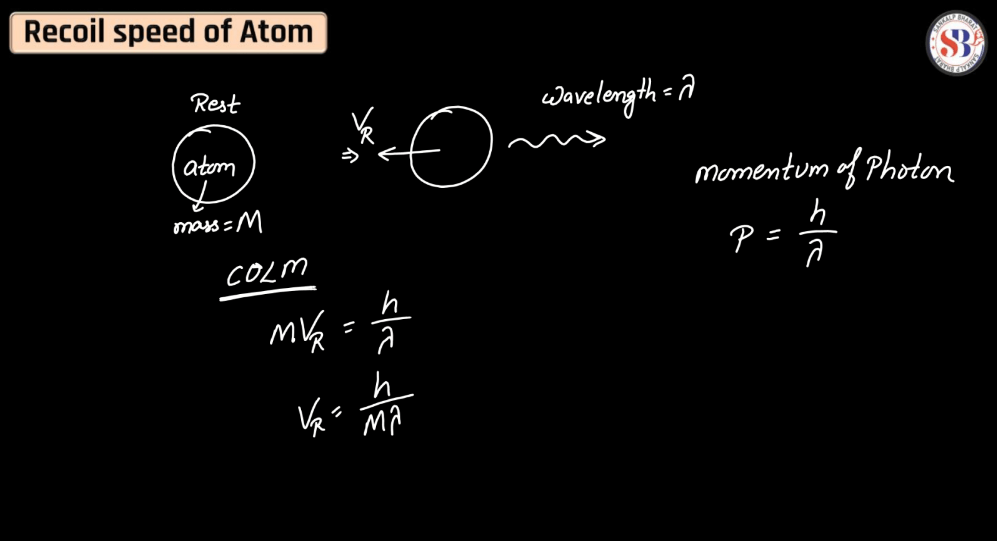
Recoil Speed of an Atom
The recoil speed of an atom refers to the velocity it acquires in response to emitting or absorbing a photon. According to the conservation of momentum, when an atom emits a photon, it experiences a recoil, propelling it in the opposite direction. This phenomenon is crucial in various scientific contexts, such as laser cooling techniques, where precise control of atomic motion is essential. Understanding recoil speed helps researchers manipulate atomic behavior, leading to advancements in fields like quantum optics and atomic physics.
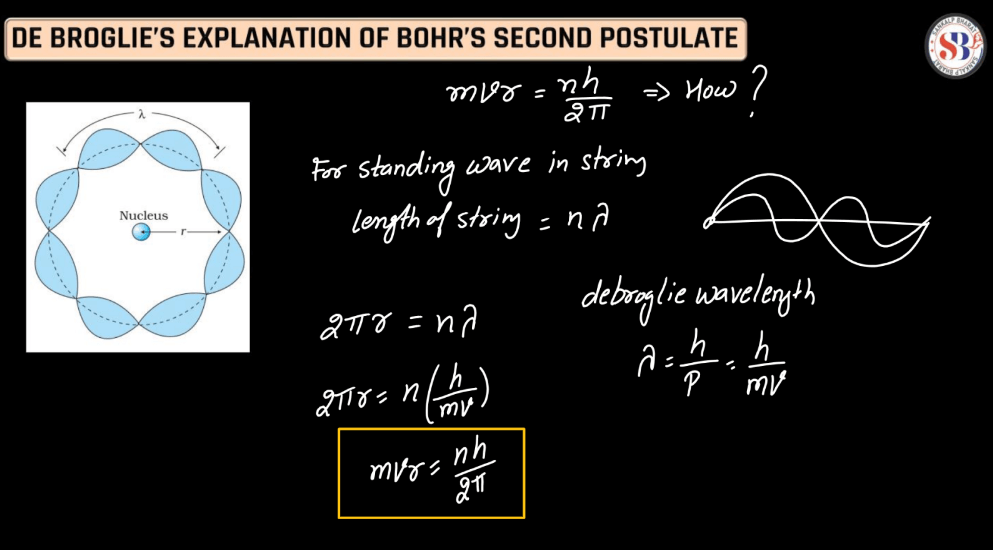
De Broglie’s Explanation of Bohr’s Second Postulate
Louis de Broglie’s explanation of Bohr’s second postulate builds on the idea that electrons exhibit both particle and wave characteristics. De Broglie proposed that electrons, in their orbit around the nucleus, behave like standing waves. These waves must complete a whole number of wavelengths to form stable orbits, aligning with Bohr’s quantized angular momentum. This concept helped reconcile classical mechanics with quantum theory, providing a wave-particle duality perspective to understand the quantization of electron orbits in Bohr’s model. De Broglie’s wave-particle duality laid the foundation for the development of quantum mechanics, shaping our understanding of the microscopic world.
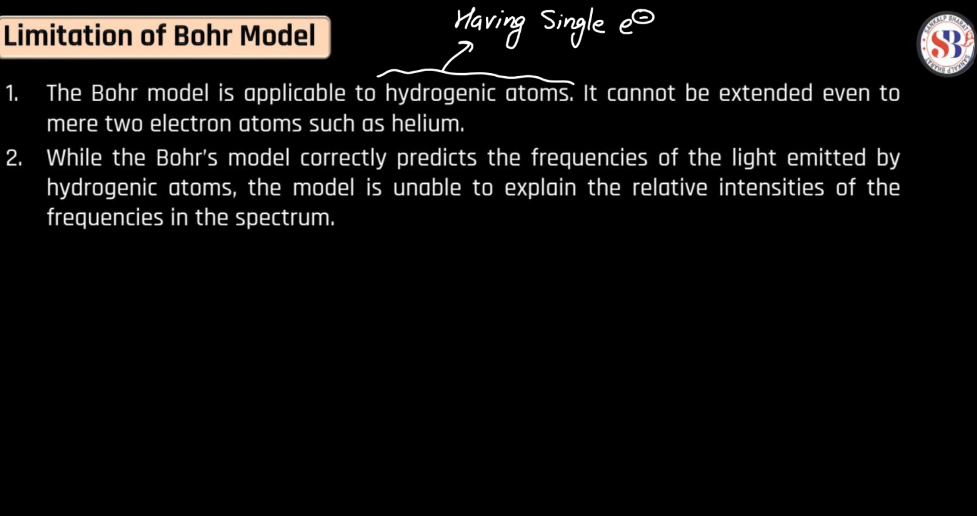
Limitations of Bohr’s Model
The Bohr Model, proposed by Niels Bohr in 1913, successfully explained some aspects of hydrogen’s spectral lines but has notable limitations. It doesn’t account for the spectral lines of multi-electron atoms, as it simplifies complex electron-electron interactions. Additionally, it doesn’t address the inherent wave-particle duality of electrons and fails to explain the finer details of atomic structure. The model’s fixed orbits conflict with the uncertainty principle, a fundamental concept in quantum mechanics. Despite its historical significance, the Bohr model is now considered a simplified representation, and more sophisticated models, like the quantum mechanical model, have since provided a more comprehensive understanding of atomic behavior.


 Ohm's Law: Definition, Formula, Limitati...
Ohm's Law: Definition, Formula, Limitati...
 Newton's First Law of Motion: Definition...
Newton's First Law of Motion: Definition...
 Kepler's Laws of Planetary Motion: First...
Kepler's Laws of Planetary Motion: First...













