Define Periodic Table
A periodic Table is a chart that organizes all known chemical elements based on their properties. Elements are substances made up of one type of atom. The Periodic table arranges elements by increasing atomic number, which is the number of photons in an atom’s nucleus. Elements in the same column share similar chemical behaviors. Each element is represented by a symbol, often derived from its name. The periodic table helps scientists predict an element’s characteristics and its reactions with other elements.
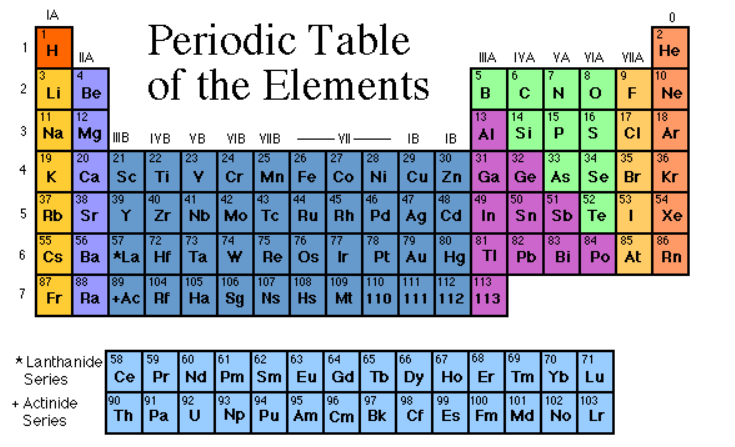
The table’s rows are called periods, and represent the energy levels of the elements, while the columns, called groups, highlight shared traits. Hydrogen and Helium start things off, and as you move across, elements get heavier. The periodic table helps scientists predict how elements will interact and combines chemistry knowledge into a neat, organized chart that simplifies the complexity of the building blocks of matter.
History of Periodic Table
The periodic table is like a giant puzzle that helps scientists organize and understand all the elements in the universe. It began in the 1860s when Dmitri Mendeleev, a Russian scientist, noticed that if he arranged elements by their properties, a pattern emerged. He created a table with rows and columns, placing elements with similar traits together. Over time, scientists discovered more elements, and the periodic table grew. Eventually, they understood that the table’s structure reflected the building blocks of matter. Today, the periodic table is a crucial tool in Chemistry, guiding scientists to predict how different elements will behave and allowing us to unlock the secrets of the elements that make up everything around us.
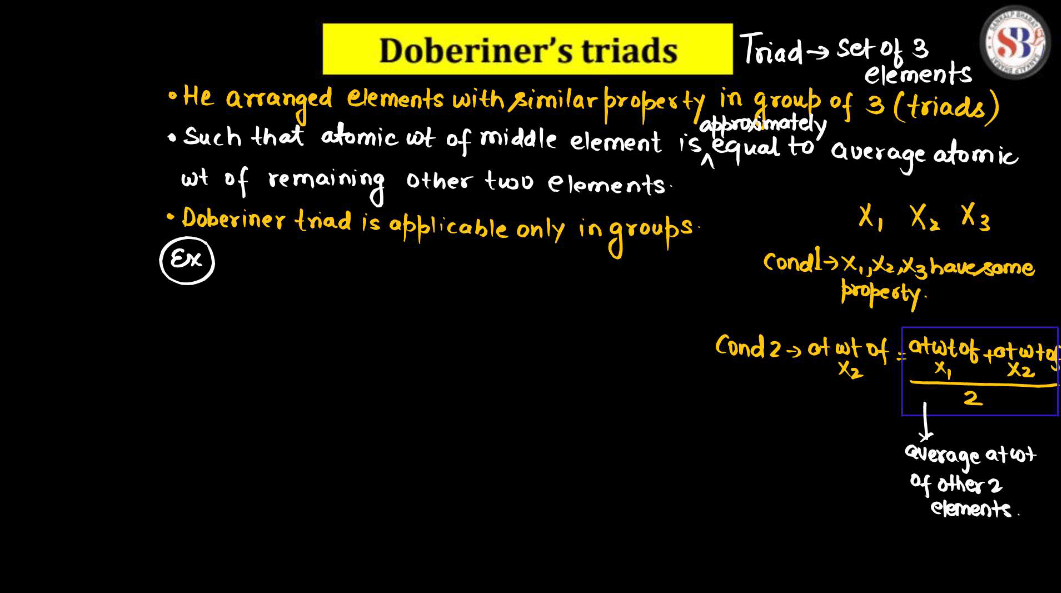
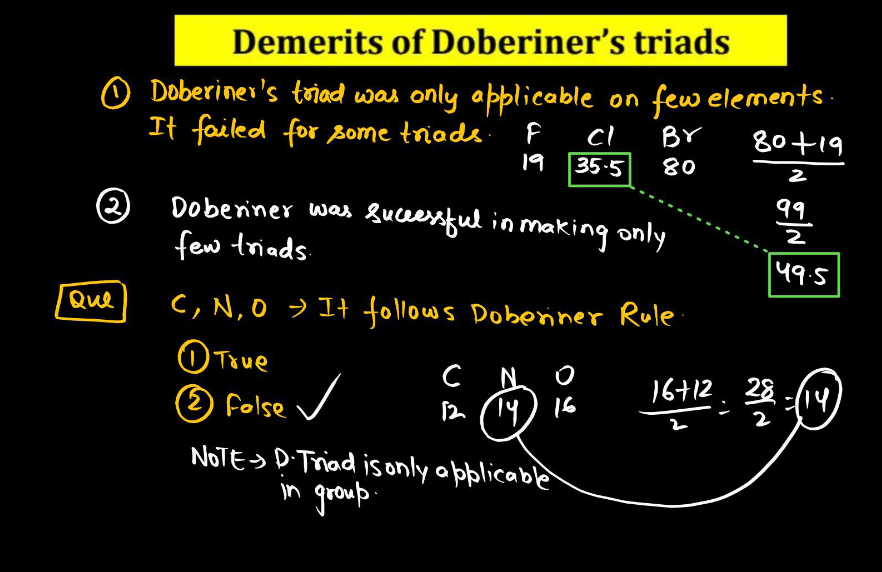
Johann Dobereiner Traits
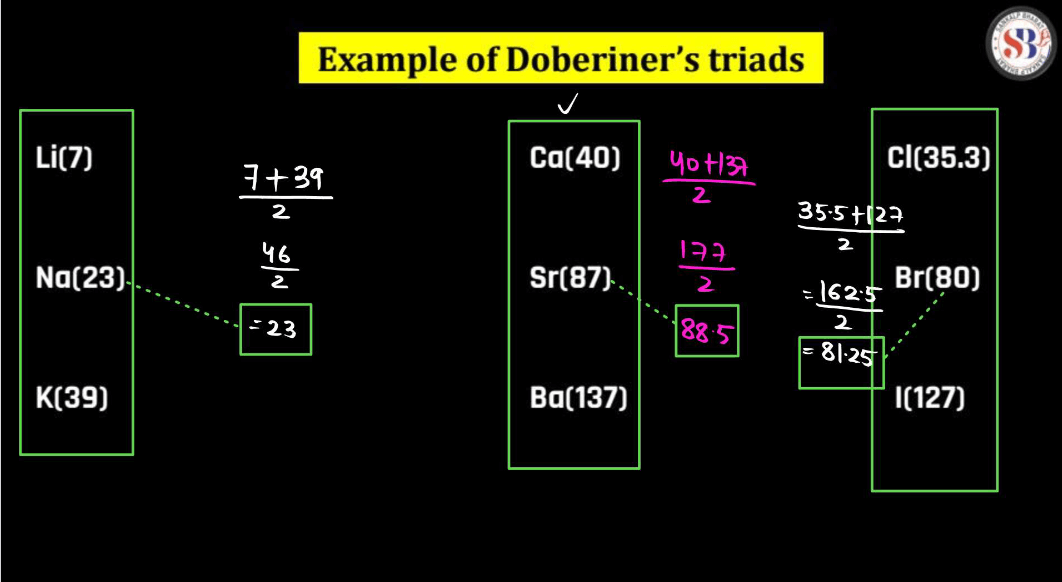
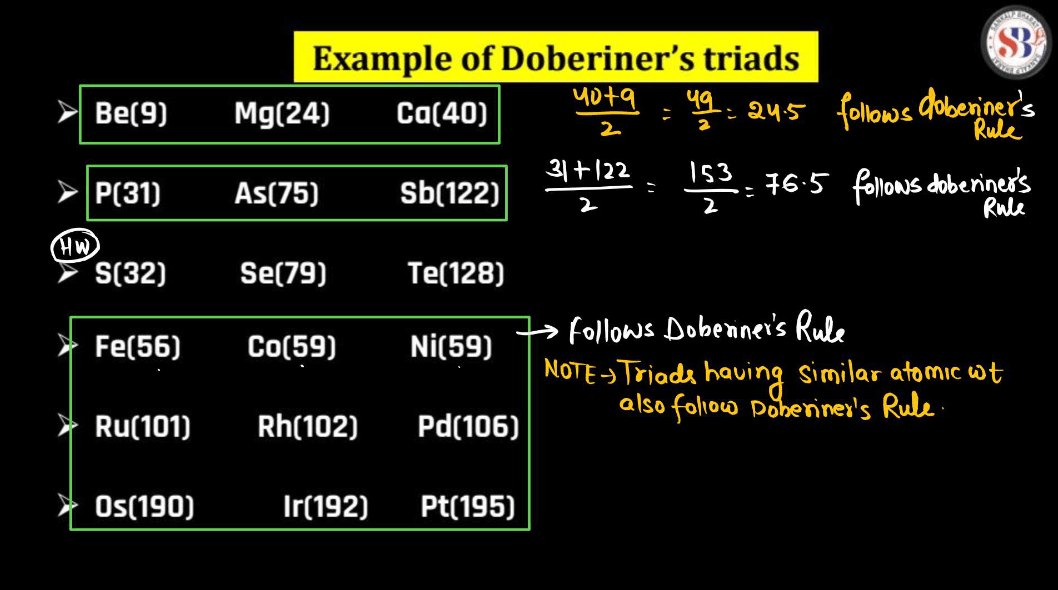
Johann Dobereiner, a German chemist in the 19th century, had a notable idea called “Triads”. He noticed that certain groups of three elements shared similar traits. In his triads, the middle element had properties that were an average of the other two. For instance, Dobereiner grouped chlorine, bromine, and iodine together, and their atomic masses formed a pattern. This concept helped lay the groundwork for the periodic table. Despite being a simple idea, Dobereiner’s triads contributed to our understanding of chemical relationships and paved the way for Dmitri Mendeleev’s later development of the periodic table, which organizes elements based on their properties.
For example:
The atomic weight of Na
(Atomic weight of Li + Atomic weight of K )/2
= (7+39)/2
=23
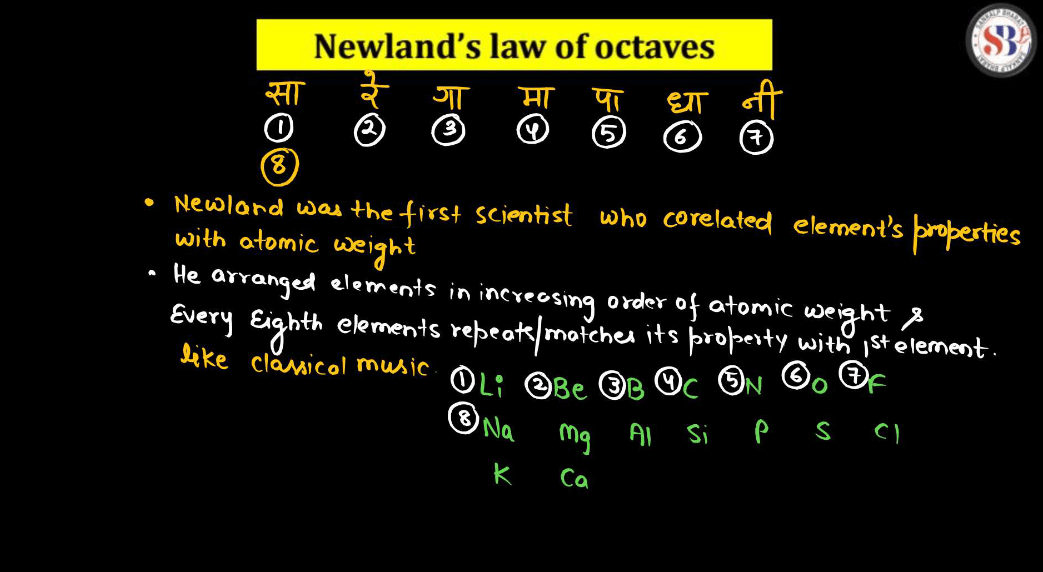
Newlands Octaves
Newlands’ octaves were an early attempt to organize elements in the periodic table. Imagine arranging elements like notes on a musical scale, where every eighth element behaves similarly. Newlands noticed a repeating pattern in every seventh element, similar to an octave in music. However, his idea faced criticism because it didn’t hold true for all elements. Still, Newland’s contribution laid the groundwork for future developments in understanding the periodicity of elements. Think of it as trying to create a melody with elements, but scientists later fine-turned the composition with more accurate arrangements. In essence, while Newland’s octave didn’t perfectly harmonize, they paved the way for the more precise periodic table we use today.

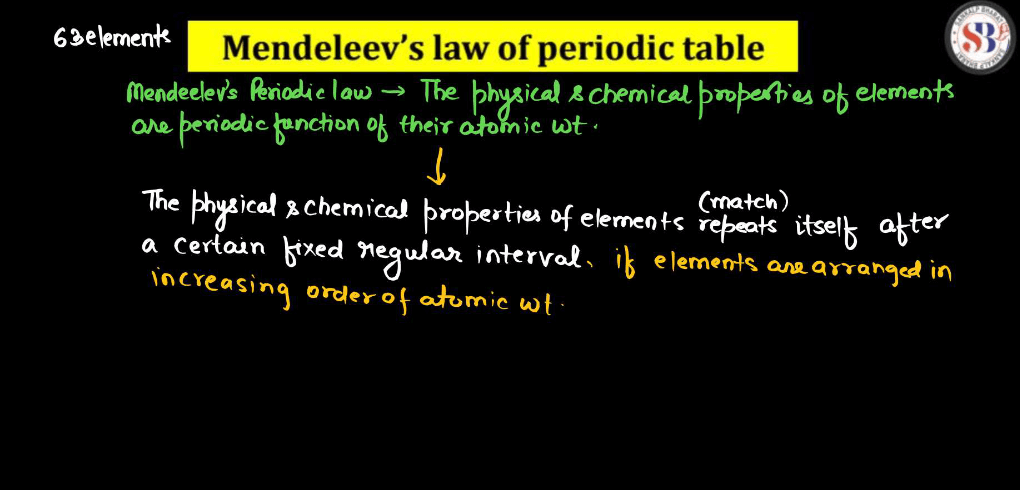
Mendeleev’s Periodic Table
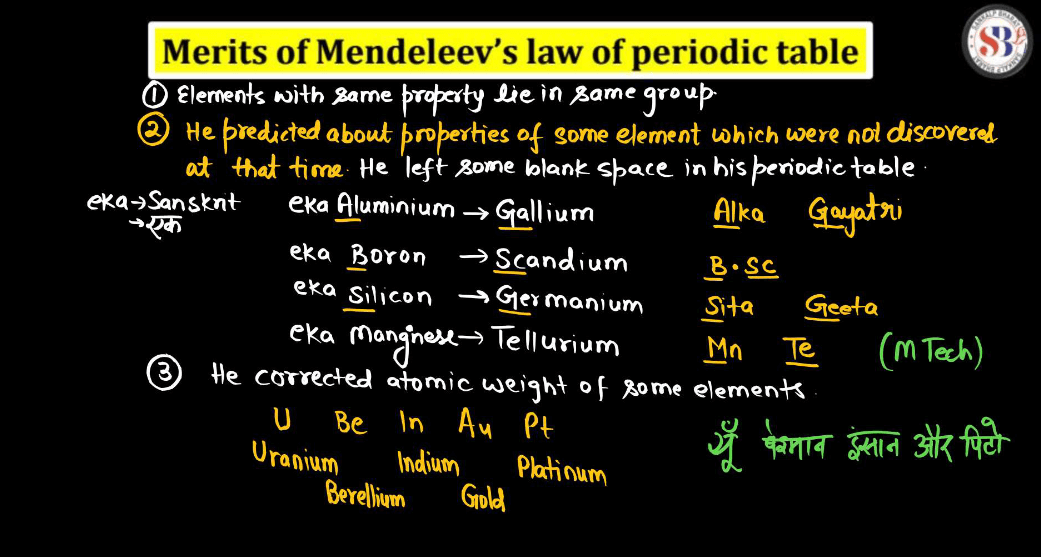
Imagine Mendeleev’s Periodic table like a big, organized grocery list for all the tiny building blocks of matter. Mendeleev noticed that when he arranged these elements in order of their atomic weights, a pattern emerged. Elements with similar properties lined up in rows and columns. This helped scientists predict the properties of missing elements. For instance, there was a gap for germanium, but Mendeleev predicted its existence and even described its properties accurately before its discovery. It’s like guessing a missing ingredient in a recipe and getting it right! Mendeleev’s periodic table laid the foundation for our modern version, making it easier for scientists to understand and work with the diverse world of elements.
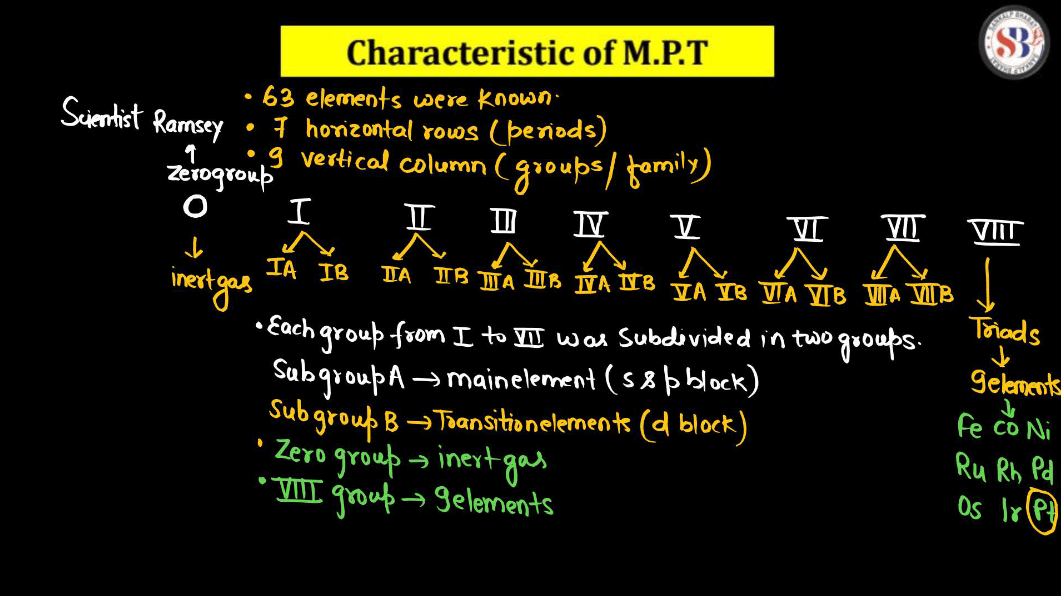
Limitations of Mendeleev’s Periodic Table
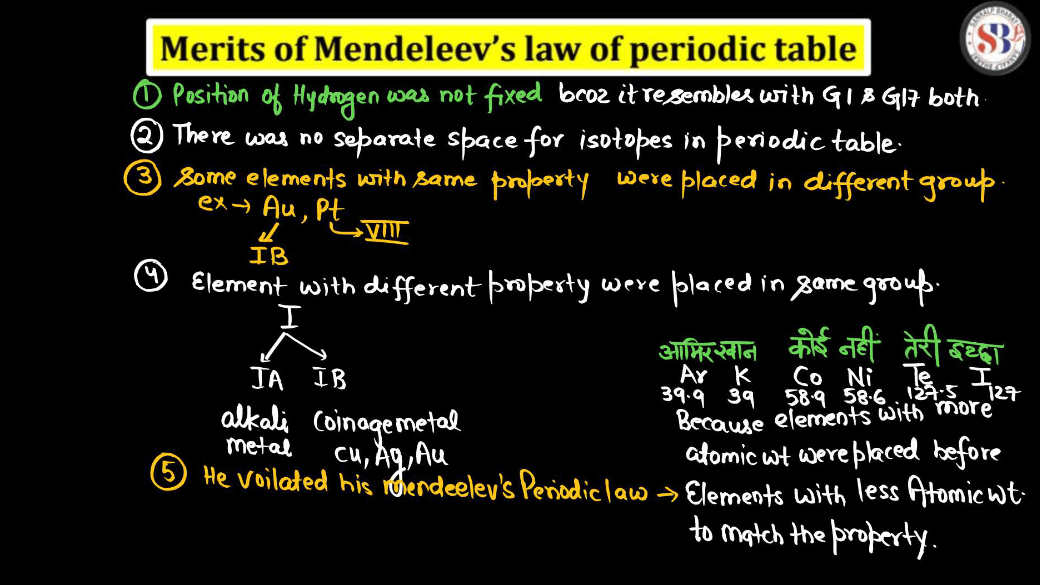
- Mendeleev left gaps in his periodic table for yet-to-be-discovered elements, assuming they would fill those spaces. This approach was a limitation as he couldn’t predict the properties of missing elements accurately.
- Some elements were placed out of order by atomic mass to align with similar chemical properties. This deviation from the strict atomic mass order was a limitation.
- Mendeleev’s table didn’t account for isotopes, elements with the same atomic number but different masses, which became evident as more isotopes were discovered.
- The periodicity of elements based solely on atomic masses was not effectively explained by Mendeleev’s table, limiting its ability to provide a comprehensive understanding of the relationships between elements.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













