Ionization energy is like the strength it takes to pull a sticker off a surface. Each electron in an atom is like a sticker stuck to an atom. The ionization energy is the energy needed to peel off one of these stickers. Some stickers come off easily, while others are stuck on tightly. Elements with low ionization energy have easily removable stickers (Electrons), while those with high ionization energy have stickers that are hard to peel off. When an electron is removed, the atom becomes positively charged. Understanding ionization energy helps scientists predict how atoms will react with each other and how they conduct electricity.
Define Ionization Energy
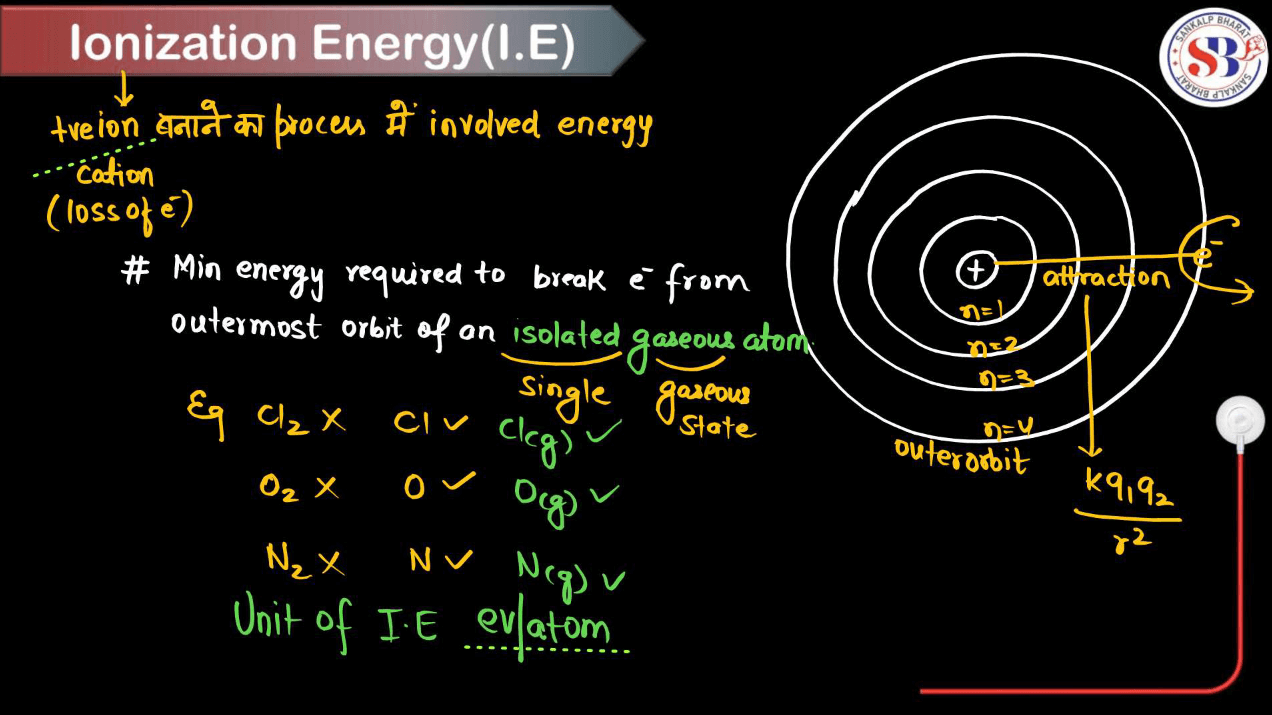
Ionization energy, also known as Ionization Potential is a concept in chemistry, that refers to the energy required to remove an electron from an atom or molecule in its gaseous state. It’s measured in Kilojoules per mole (kJ/mol). Imagine electrons as kids playing around an atom, each kid holding onto the atom like a handhold on a merry-go-round. Ionization energy is like the strength it takes to pull a kid off the merry-go-round.
Some kids let go easily (low ionization energy), while others hold on tight (high ionization energy). Factors affecting the ionization energy include atomic size (smaller atoms have higher ionization energy), electron shielding (more shielding decreases ionization energy), and electron-electron repulsion (more repulsion increases ionization energy). Ionization energy trends across the periodic table: it generally increases from top to bottom. This trend helps predict how atoms bond, react, and form ions in chemical reactions.
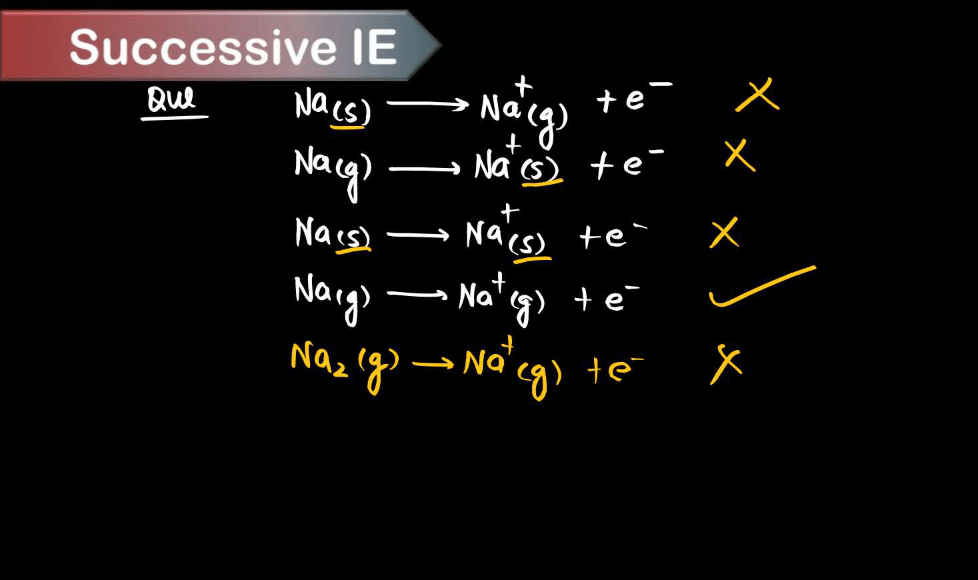
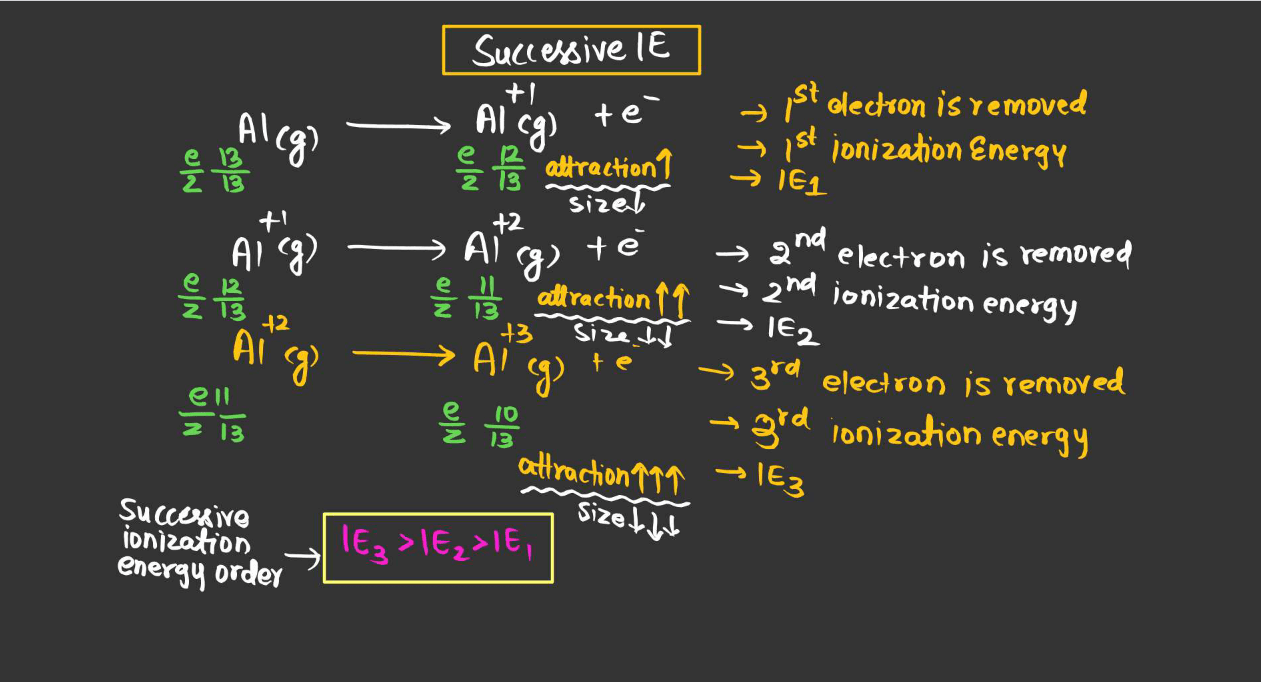
Factors Affecting Ionization Energy
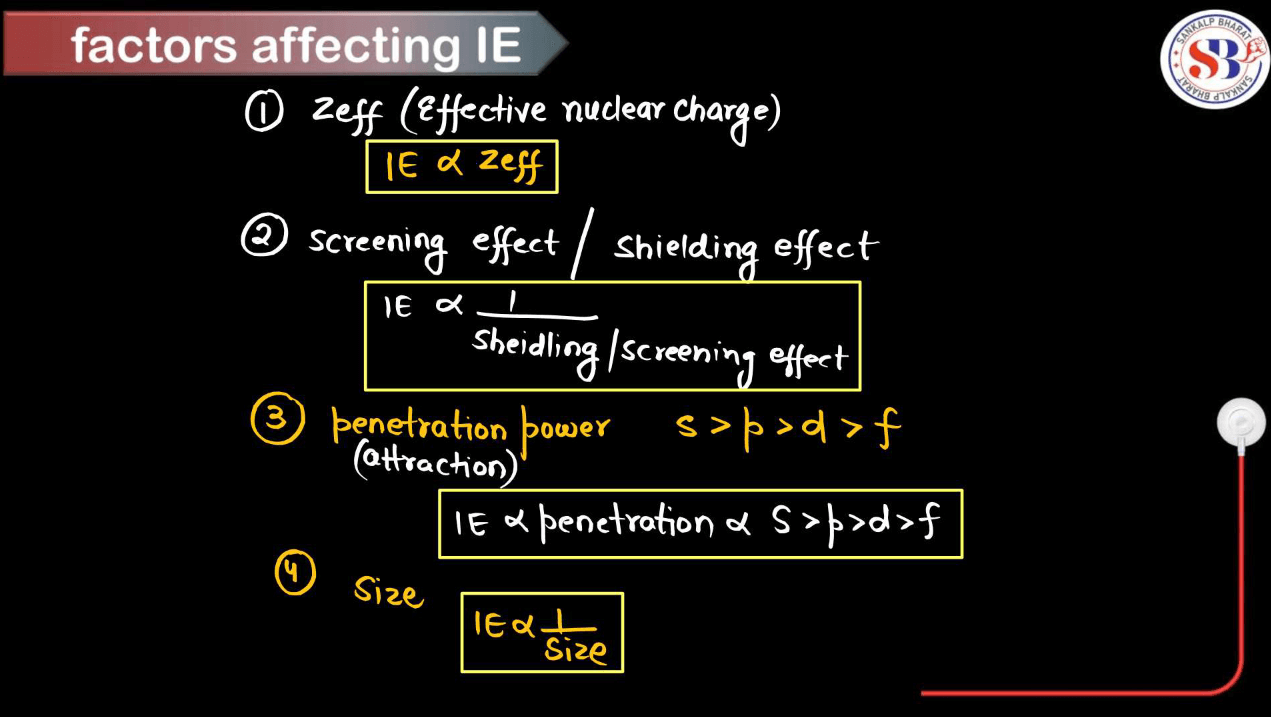
Ionization energy, the energy required to remove an electron from an atom or ion in its ground state, is influenced by several factors. These factors collectively determine the ionization energy of an atom.
- Atomic Size: Generally, the larger the atomic size, the lower the ionization energy. This is because electrons are further away from the nucleus in larger atoms, so they experience weaker attraction, making them easier to remove.
- Nuclear Charge: Higher nuclear charge (more protons in the nucleus) increases the attraction for electrons, leading to higher ionization energy.
- Electron shielding: Inner shell electrons shield the outer electrons from the full effect of the nuclear charge, reducing the effective nuclear charge experienced by outer electrons and thereby lowering ionization energy.
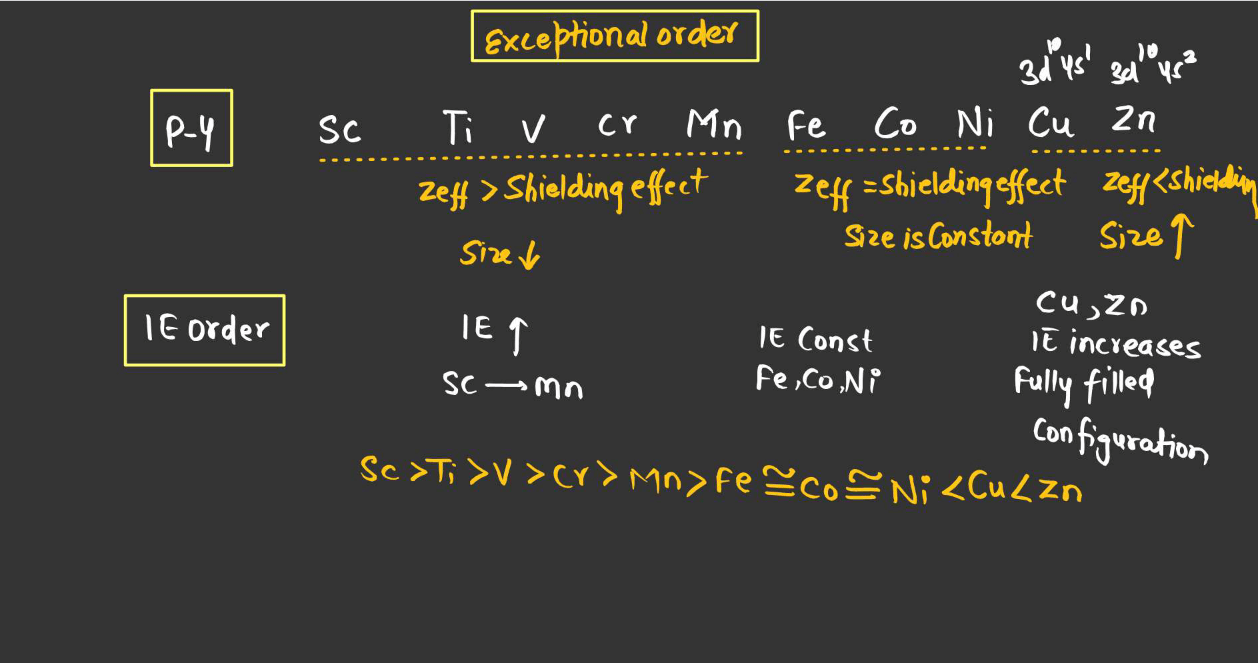
- Subshell and Orbital: Electrons in higher energy orbitals are easier to remove than those in lower energy orbitals within the same shell due to differences in electron-electron repulsion.
- Penetration of Orbitals: Electrons in orbitals with higher penetration into the nucleus experience greater nuclear attraction, leading to higher ionization energy.
- Electron-electron repulsion: Electrons repel each other, making it easier to remove an electron from a partially filled subshell than from a fully filled subshell due to electron-electron repulsion.
Trends of Ionization Energy in the Periodic Table
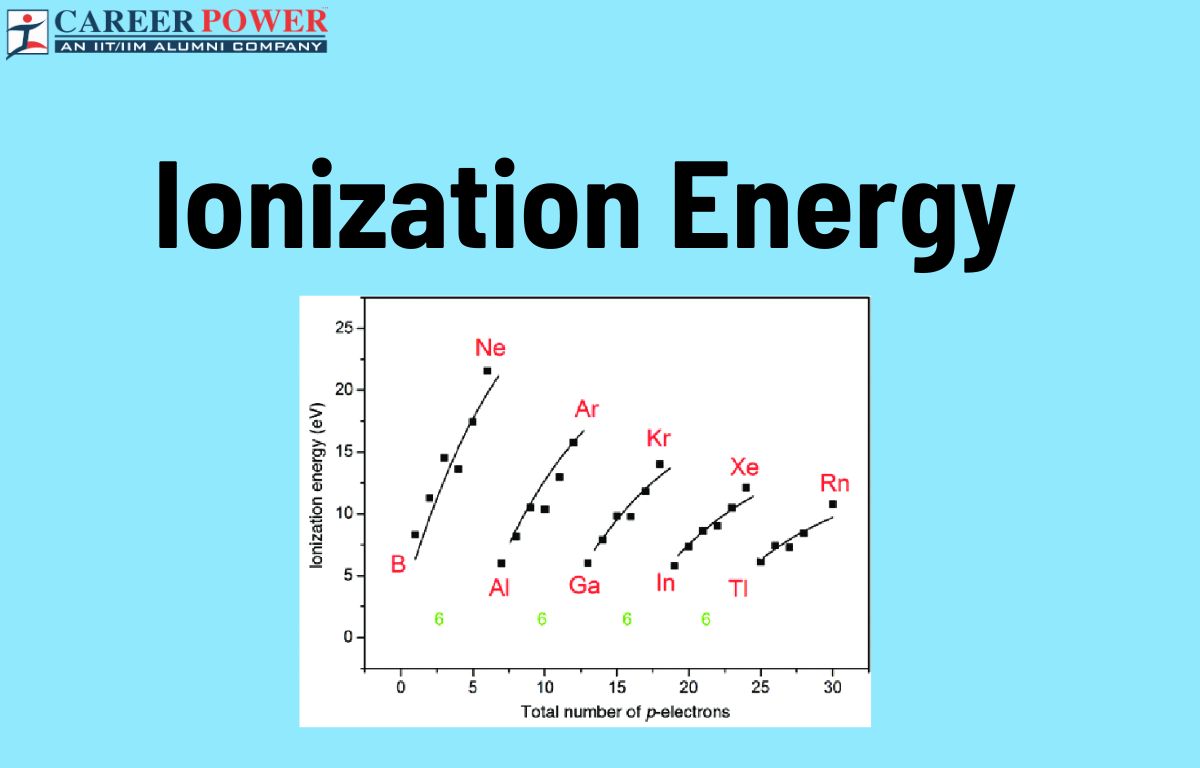
Ionization energy, often denoted as IE, is the energy required to remove an electron from a gaseous atom or ion. In the periodic table, ionization energy generally follows several trends. Understanding these trends helps predict and interpret the behavior of elements regarding their ionization energy, which is crucial in various chemical reactions and properties.
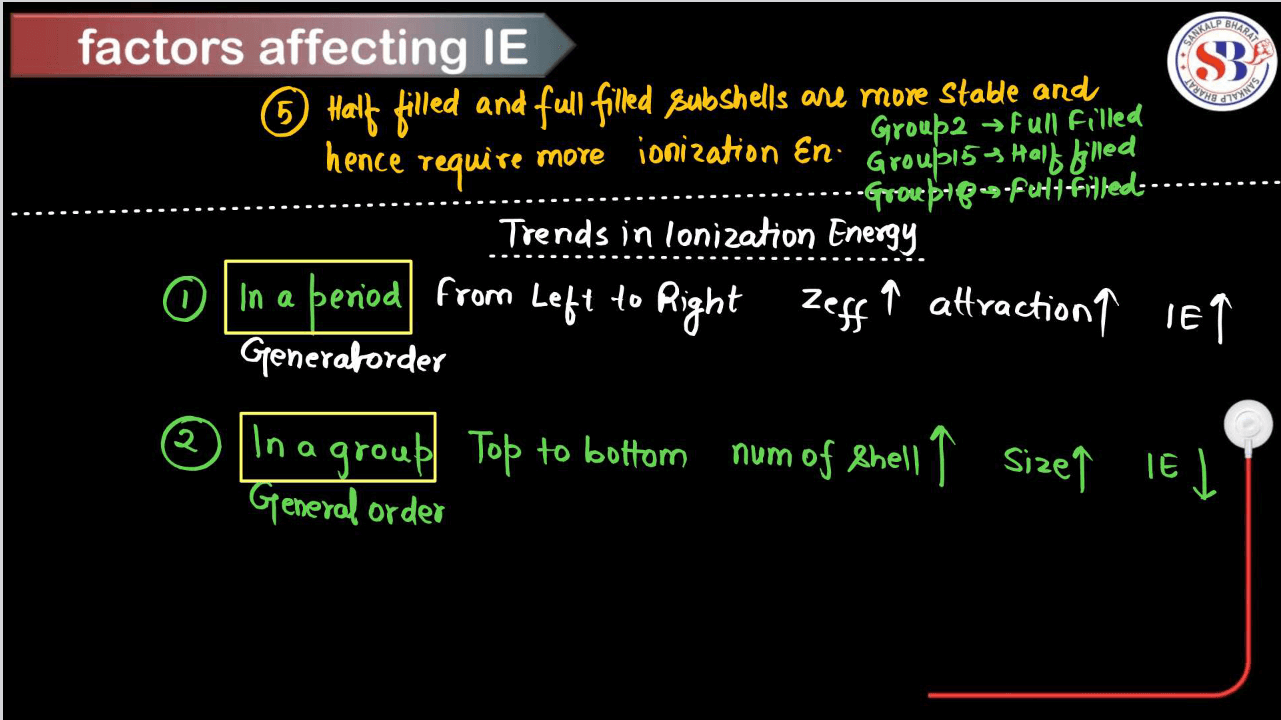
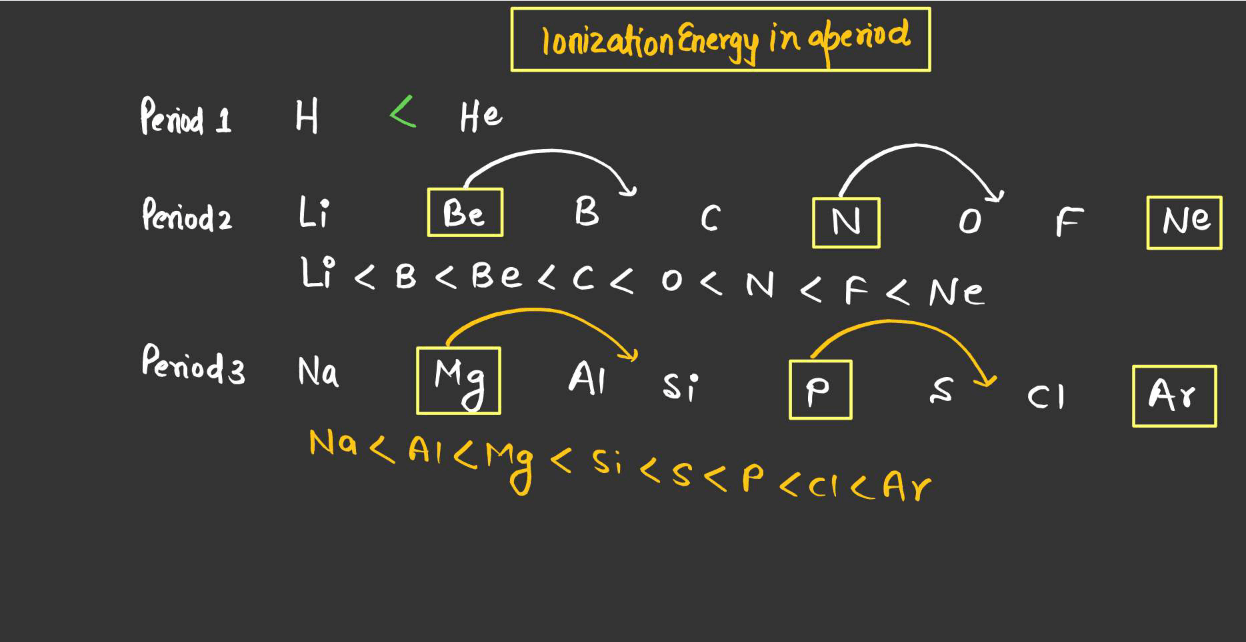
- Periodic Trend Across a Period (From Left to Right)
Ionization energy generally increases across a period. This is because, as you move from left to right across a period, the effective nuclear charge (the positive charge experienced by an electron) increases due to the increasing number of photons in the nucleus. This stronger attraction between the positively charged electrons requires more energy to remove an electron, thus increasing ionization energy.
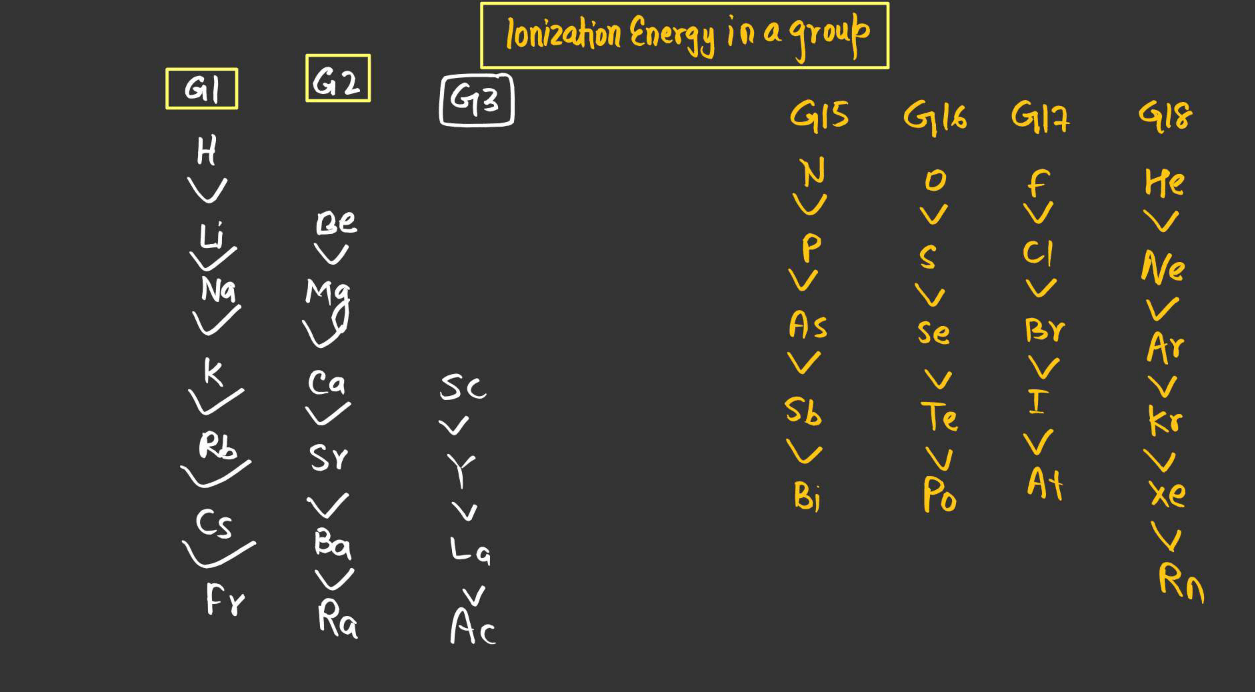
- Periodic Trend Down a Group (From top to bottom):
Ionization energy generally decreases down a group. As you move down a group, the number of electron shells increases, leading to a greater distance between the outermost electrons and the Nucleus. This results in weaker attractive forces between the nucleus and the outermost electrons, making it easier to remove an electron and thus decreasing ionization energy.
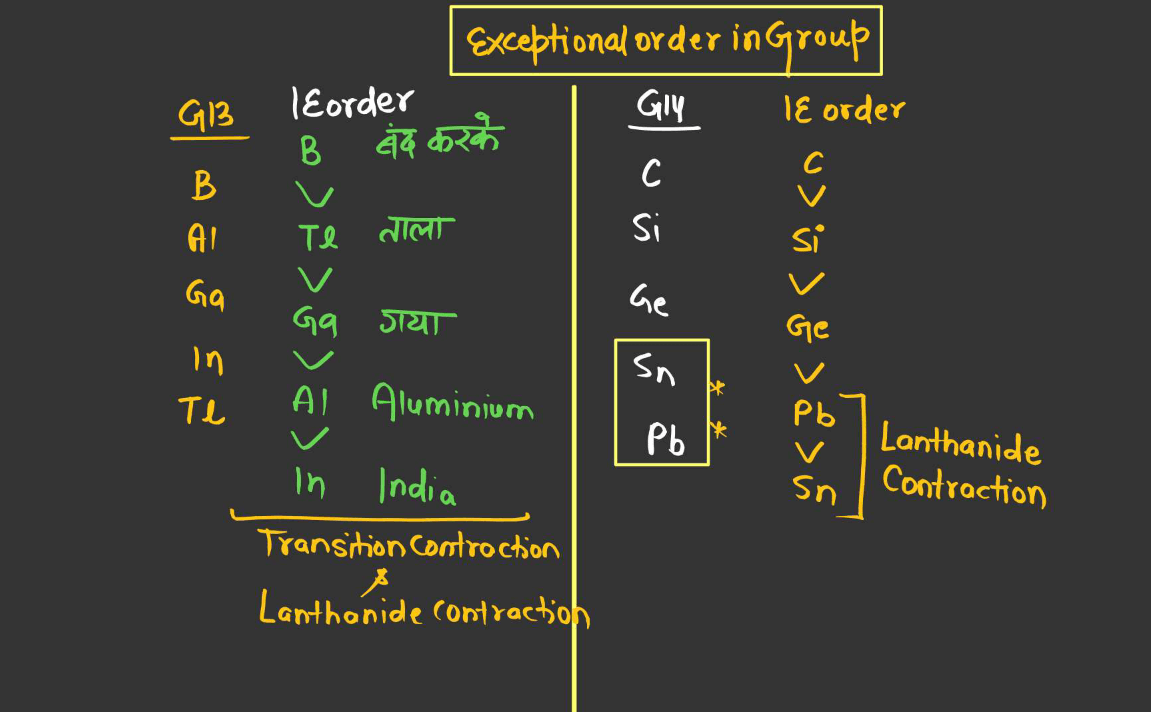
- Exception: Group 2 Elements:
There’s an exception to the general trend of decreasing ionization energy down a group with the Group 2 elements (alkaline earth metals), such as beryllium (Be), magnesium (Mg), calcium (Ca), etc. While ionization energy generally decreases down a group, there’s a slight increase in ionization energy from one element to the next within Group 2. This is due to the added stability associated with having a completely filled s-subshell in the preceding noble gas configuration, resulting in higher ionization energy compared to the elements above it in the group.
How to Determine the Ionization Energy of an Element?
Ionization energy is the energy needed to remove an electron from an atom. To determine the ionization energy of an element, scientists use a method called spectroscopy. They shine high-energy light, like ultraviolet or X-rays, onto atoms of the elements. When an electron absorbs this energy, it gets excited and may leave the atom, creating a positively charged ion. By measuring the energy required to remove each electron, statistics can determine the ionization energy levels of the element. The first ionization energy is the energy needed to remove the outermost electron, while subsequent ionization energies remove inner electrons. Higher ionization energies indicate stronger binding between electrons and the nucleus. This information is crucial for understanding an element’s chemical behavior and its placement in the periodic table.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













