Mixtures are a fundamental concept in the field of chemistry. Mixtures are combinations of two or more substances that are physically intermingled but not chemically bonded together. There are 2 types of mixtures namely Heterogeneous Mixture and Homogeneous Mixture. Understanding mixtures is a fundamental aspect of chemistry as it plays a crucial role in various chemical processes and reactions. Here in the article, we have briefly discussed about homogeneous and heterogeneous mixtures, their differences, and their importance.
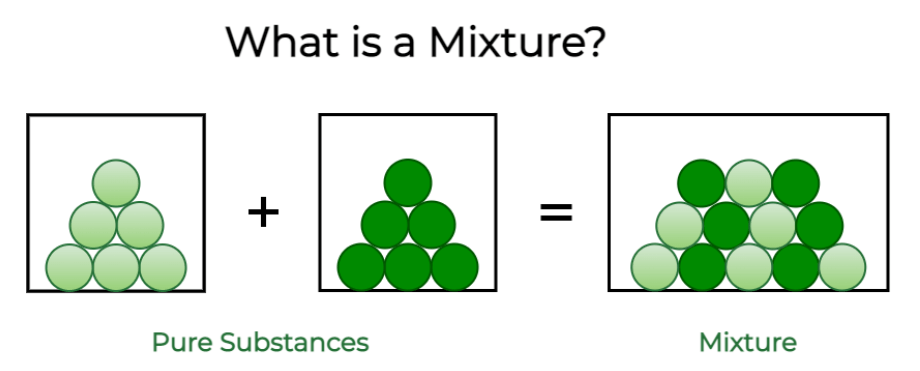
What is a Mixture?
“Mixture” refers to a physical combination of two or more different substances or elements that are physically intermingled but not chemically bonded. These substances are typically in varying proportions and can be separated by physical means, such as filtration, distillation, or evaporation. Mixtures can be homogeneous mixture and heterogeneous mixture. Homogeneous, where the components are evenly distributed (like a solution), or heterogeneous, where the components are not evenly distributed (like a salad). Some examples of Mixture include solutions, suspensions, and colloids.
Examples of Mixtures
Here we have mentioned a few examples, but mixtures can be found in many aspects of daily life and can vary widely in composition and properties.
- Soil: A mixture of minerals, organic matter, water, and oil found in the Earth’s crust.
- Ink: A mixture of pigments, solvents, and other chemicals used for writing or printing.
- Fruit Juice: A mixture of water and the juices extracted from fruits.
- Salad: A mixture of various vegetables, sometimes with fruits, dressing, and other toppings.
- Saltwater: A mixture of salt (solute) dissolved in water (solvent).
- Trail Mix: A combination of nuts, dried fruits, and sometimes chocolate or candies.
- Air: A mixture of gases, primarily nitrogen, oxygen, carbon dioxide, and others, that makes up Earth’s atmosphere.
- Cereal: A mixture of grains, often with added vitamins and flavors, used for breakfast.
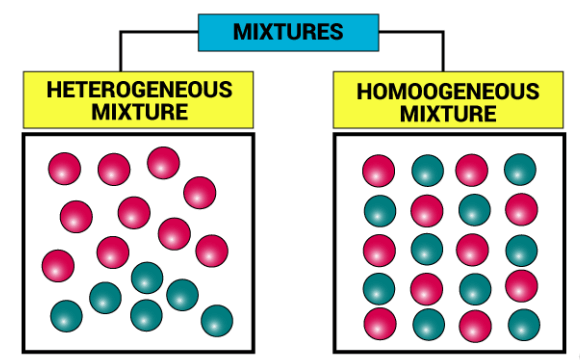
Types of Mixtures
There are two types of mixtures: Homogeneous mixture and Heterogeneous mixture. Both mixtures have many different characteristics and features. These mixtures are characterized by the degree of uniformity in their composition.
| Different Types of Mixtures | ||
| Types of Mixtures | Characteristics | Examples |
| Homogeneous Mixture | Homogeneous mixtures are uniform compositions at the molecular level. | Examples are saltwater. |
| Heterogeneous Mixture | Heterogeneous mixtures are non-uniform compositions with visible different phases. | Salad depressing (oil and vinegar), bowl of cereal, and milk. |
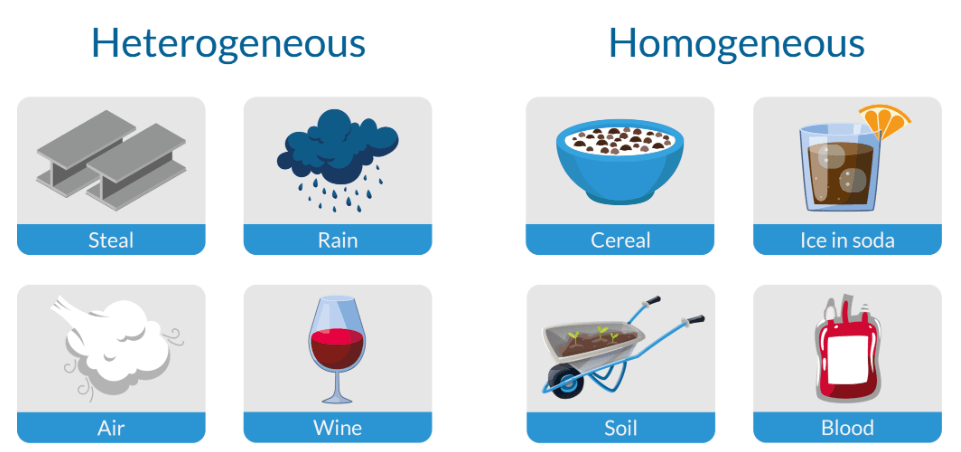
What is a Heterogeneous and Homogeneous Mixture?
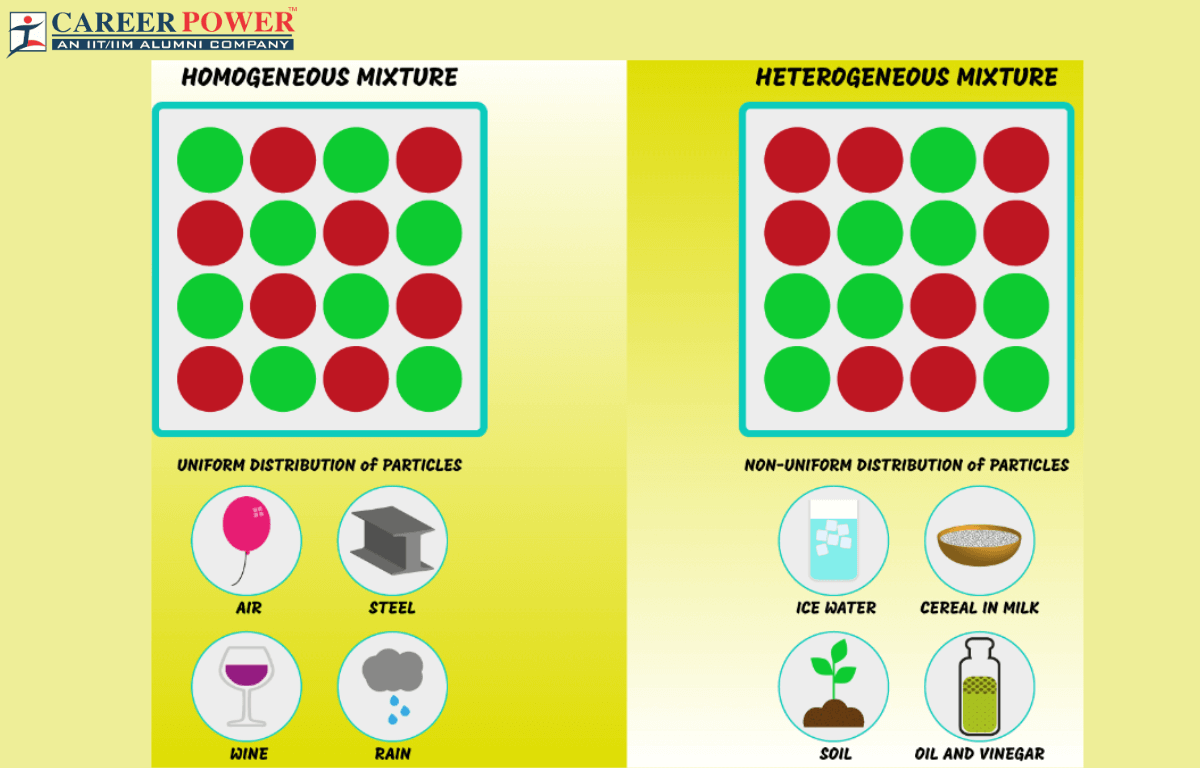
Heterogeneous and homogeneous mixtures are two different types of mixtures in chemistry. Homogeneous mixtures are uniform and have the same composition, while heterogeneous mixtures are non-uniform and consist of visibly different components.
Homogeneous Mixture
A homogeneous mixture is a uniform combination of two or more substances, where the components are evenly distributed at a molecular or microscopic level. This results in a consistent composition and properties throughout the mixture, making it difficult to distinguish individual substances. An example of a homogeneous mixture is a solution, like salt dissolved in water, where you can’t distinguish the salt particles from the water molecules.
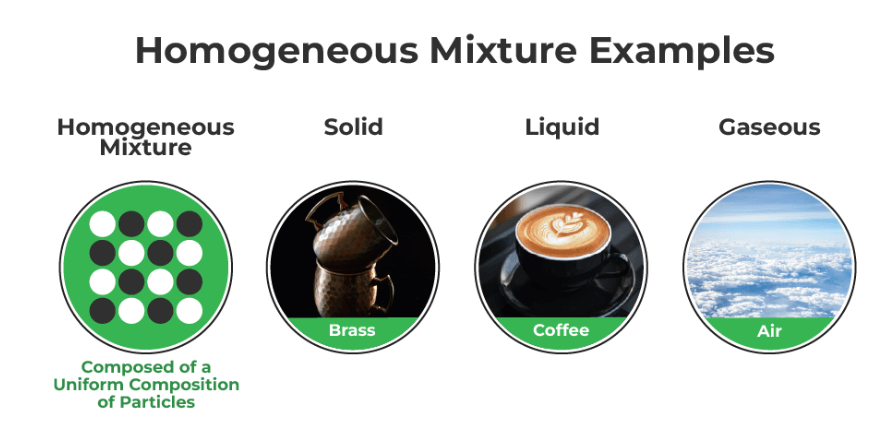
- A homogeneous mixture is also known as a solution.
- The homogeneous mixture appears uniform throughout, with the same composition and properties in all parts.
- A homogeneous mixture will remain uniform over time as long as no chemical reactions occur between its components.
- The concentration of a solute in a homogeneous mixture can vary, with terms like “dilute” and “concentrated” describing the amount of solute present in the solution.
- Examples of homogeneous mixtures include saltwater (salt dissolved in water), air (a mixture of gases), and sugar dissolved in water.
Heterogeneous Mixture
A heterogeneous mixture is a combination of two or more substances in which the components are not uniformly distributed. Instead, they remain visibly separate, creating distinct regions or phases within the mixture. Examples include salad, where you can see different ingredients like lettuce, tomatoes, and croutons, or a bowl of cereal with milk; where you can see and separate the different components.
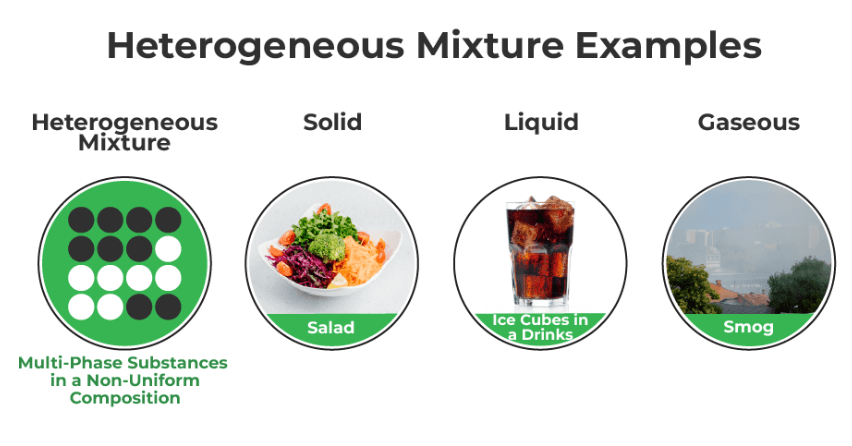
- A heterogeneous mixture is not uniform and consists of distinct, visibly different substances or phases.
- The components of a heterogeneous mixture can be separated physically.
- Heterogeneous mixtures do not remain uniform over time; the components may settle or separate further due to gravity or other factors.
- Heterogeneous mixtures are prevalent in our daily lives and in various scientific and industrial applications. They are often used for their unique properties, such as the ability to mix materials with different characteristics.
- Examples of the heterogeneous mixture include a salad with different ingredients, a mixture of oil and water, or a bowl of cereal with milk.
Difference Between Homogeneous and Heterogeneous Mixture
Homogeneous and heterogeneous mixtures are two fundamental categories in chemistry, distinguished by the uniformity of their composition. The key difference lies in the uniformity of distribution: Homogeneous mixtures are uniform throughout, while heterogeneous mixtures are non-uniform distribution and distinct regions with varying compositions.
| Difference Between Homogeneous Mixture and Heterogeneous Mixture | ||
| Characteristics | Homogeneous Mixture | Heterogeneous Mixture |
| Definition | A homogeneous mixture is a uniform composition throughout. | A heterogeneous mixture is a non-uniform composition, with distinct phases. |
| Appearance | Appears as a single, consistent phase. | Contains visibly different components. |
| Example | Examples of homogeneous mixtures are saltwater, air, and sugar dissolved in water. | Examples of heterogeneous mixtures are sand and water, oil, and water. |
| Separation | It is very difficult to separate components from a homogeneous mixture. | Components can be separated easily from a heterogeneous mixture. |
| Properties | Homogeneous mixtures have uniform properties throughout. | Properties may vary in different regions in a homogeneous mixture. |
| Particle Size | Particles are evenly distributed in a homogeneous mixture. | Particles have varying sizes and are shaped in heterogeneous mixtures. |
Importance of Homogeneous & Heterogeneous Mixture
Both homogeneous and heterogeneous mixtures have many different importance. Heterogeneous mixtures, like solutions, are vital for consistent compositions in fields such as chemistry and food preparation. Heterogeneous mixtures play a key role in geology by helping identify non-uniform substances like rocks and soil.
Importance of Homogeneous Mixture
- Uniformity: Homogeneous mixtures have a consistent composition throughout. This uniformity ensures that every part of the mixture contains the same proportion of substances, making them reliable for experiments and applications where consistency is crucial.
- Predictability: Because of their uniform nature, homogeneous mixtures allow for precise measurements and calculations. Scientists and engineers rely on them when designing and conducting experiments or when formulating products like medicines or chemicals.
- Ease of Handling: Homogeneous mixtures are generally easier to handle and transport than heterogeneous mixtures. This property simplified processes in industries like pharmaceuticals, food production, and chemicals.
- Efficient Reactions: In chemistry, homogeneous mixtures facilitate efficient chemical reactions. All reactants are evenly distributed, promoting rapid and uniform reaction, which is vital for laboratory work and industrial processes.
- Homogeneous Properties: The properties of a homogeneous mixture are uniform, which allows scientists and engineers to study and manipulate specific properties like temperature, concentration, and pressure with precision.
Importance of Heterogeneous Mixture
- Diverse Composition: Heterogeneous mixtures contain visibly distinct components or phases that are not evenly distributed. These components can have different properties, such as density, solubility, or chemical composition.
- Visual Identification: One of the defining characteristics of heterogeneous mixtures is that you can often see the different components with the naked eye. For example, a salad with lettuce, tomatoes, and croutons is a heterogeneous mixture because you can easily distinguish these components.
- Versatility: Heterogeneous mixtures are versatile and have various applications. They can be found in everyday scenarios like food preparation, geological samples, and industrial processes.
- Separation Methods: Separation techniques such as filtration, decantation, and centrifugation are commonly used to isolate the different components of a heterogeneous mixture. These techniques are essential in fields like chemistry, environmental science, and recycling.
- Culinary Arts: Cooking often involves creating and manipulating heterogeneous mixtures. Recipes combine ingredients with different properties and textures to achieve desired flavors and textures.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













