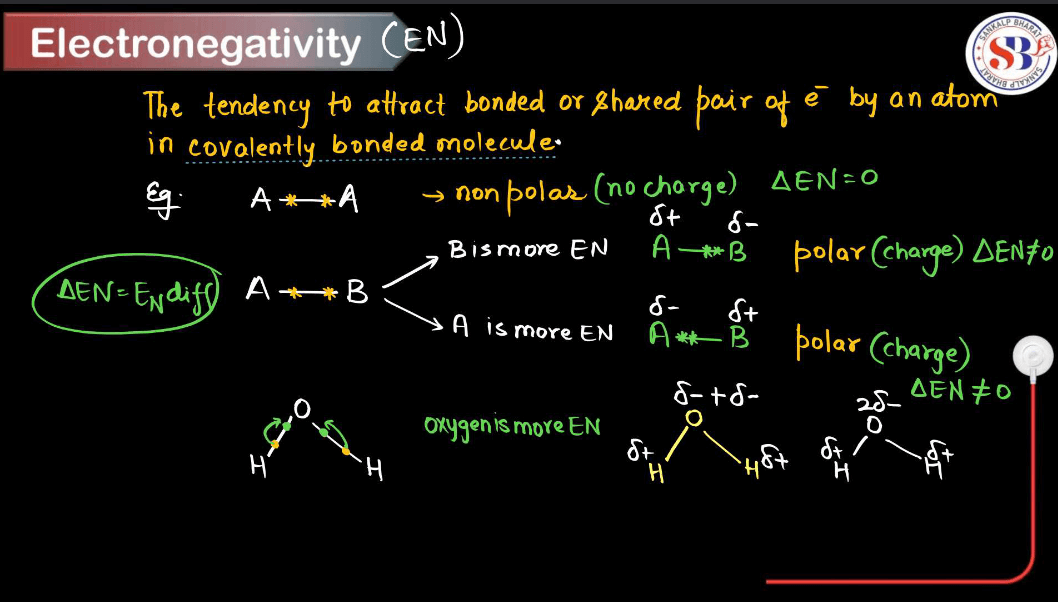
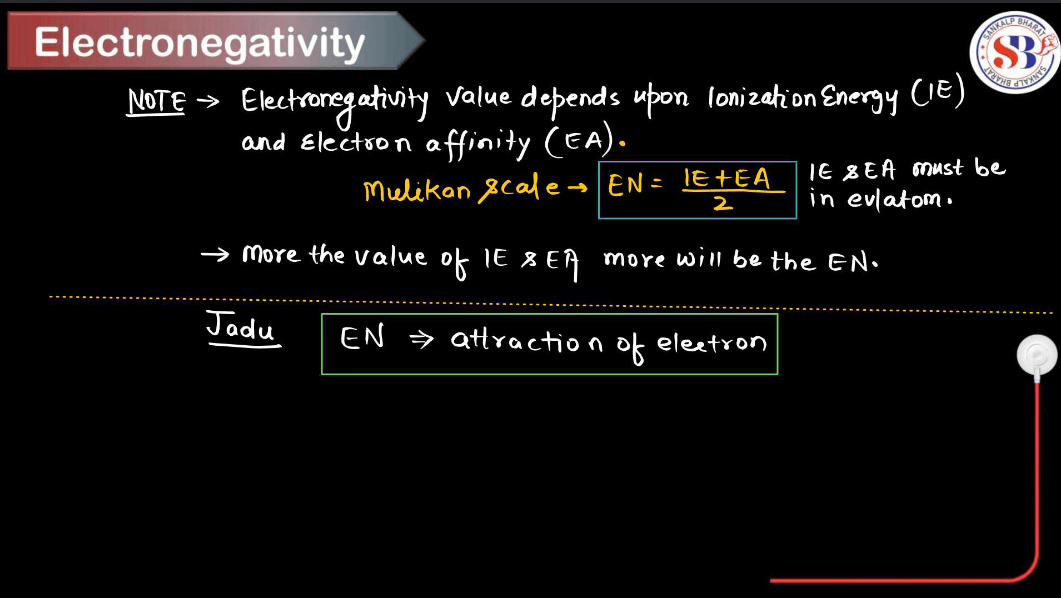
What is Electronegativity?
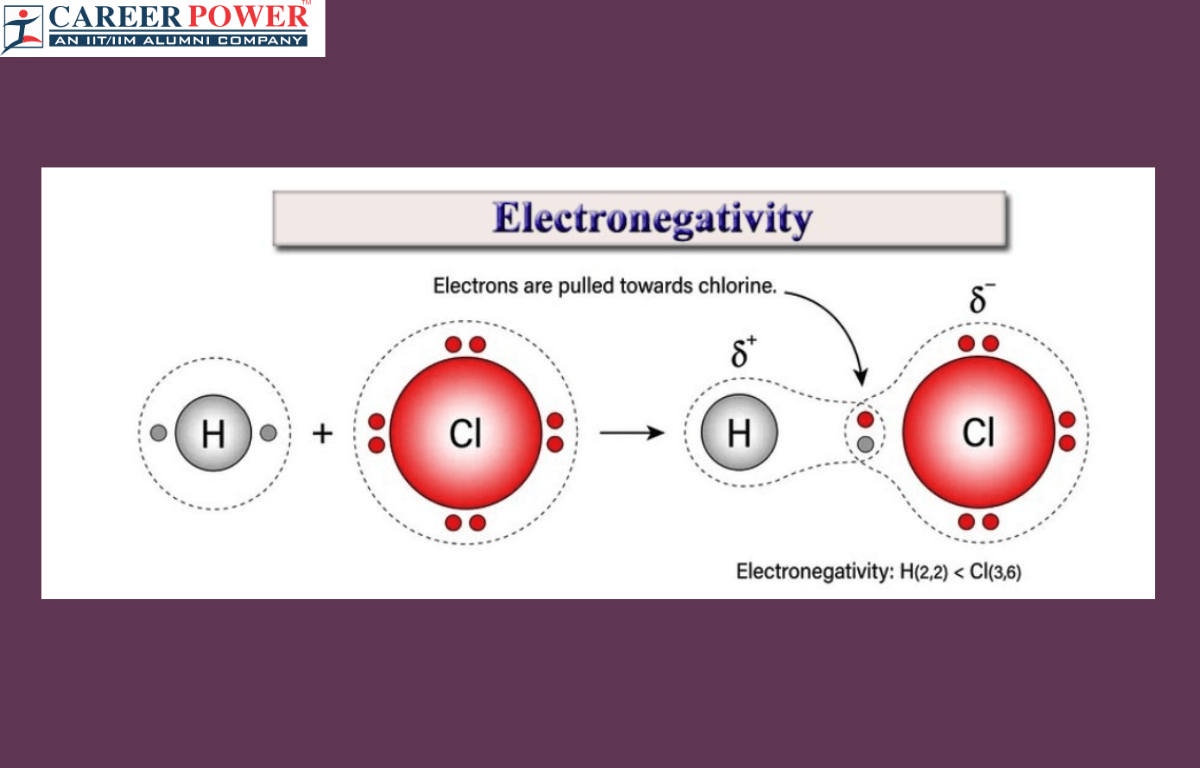
Electronegativity is a measure of how much an atom attracts electrons toward itself when it is part of a chemical bond. In simpler terms, it is like a magnetism for electrons in a molecule. Atoms with high electronegativity, like oxygen or fluorine, pull electrons strongly toward them, creating a partial negative charge on their side of the bond. On the other hand, atoms with low electronegativity, like alkali metals, don’t attract electrons as strongly and often end up with a partial positive charge.
Electronegativity helps us understand how atoms share electrons in covalent bonds. If two atoms have similar electronegativity, they share electrons equally (nonpolar bond). However, if there’s a big difference in electronegativity, one atom hogs the electrons, leading to a polar bond. This concept is crucial in predicting molecule behavior and understanding chemical reactions in chemistry.
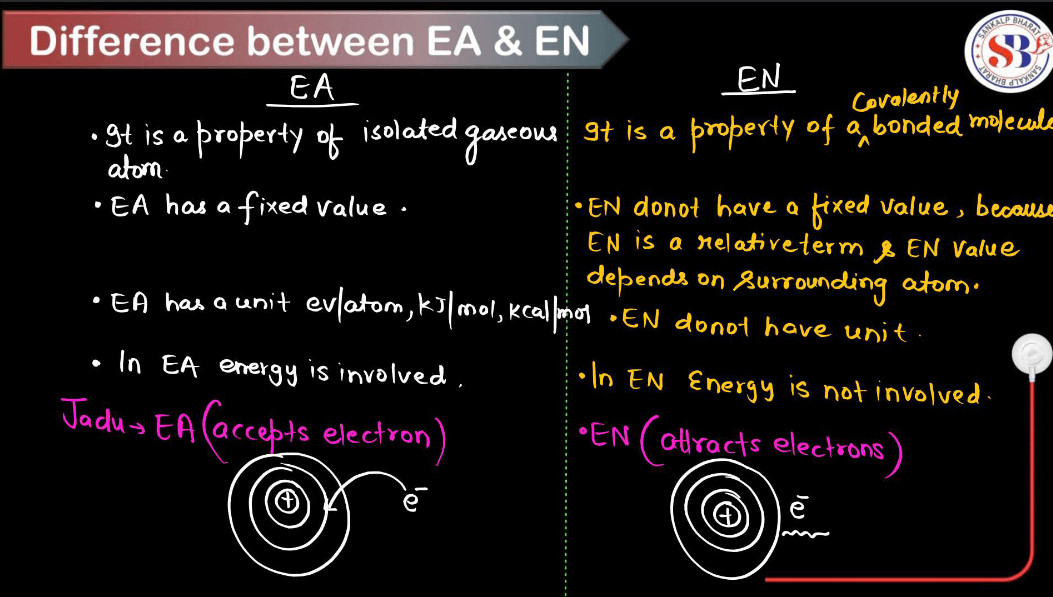
Difference Between EA and EN
The Table discussed below will highlight many differences between Electronegativity and Electron Affinity.
| Difference Between Electronegativity and Electron Affinity | ||
| Properties | Electronegativity (EN) | Electron Affinity (EA) |
| Definition | Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. | Electron affinity is the measure of the energy released or absorbed when an electron is added to a neutral atom in the gaseous state. |
| Unit | Electronegativity is a dimensionless quantity (no specific unit). | Electron affinity is measured in kilojoules per mole (kJ/mol). |
| Nature | Electronegativity is a relative concept used to compare different elements. | Electron affinity is an absolute value specific to each element. |
| Influence | Electronegativity is influenced by factors such as atomic size, nuclear charge, electron shielding, and periodic trends. | Electron affinity is influenced by electron configuration, atomic size, and nuclear charge. |
| Periodic Trend | Electronegativity generally increases from left to right across a period and decreases down a group in the periodic table. | Electron affinity also generally increases from left to right across a period but may not strictly follow a trend down a group. |
| Application | Electronegativity is widely used in predicting bond types (ionic, covalent) and polarity. | Electron affinity is important in understanding the stability and reactivity of atoms and molecules. |
| Physical Representation | Electronegativity is often depicted using Pauling electronegativity values or other scales. | Electron affinity values are represented as energy changes (positive or negative) when an electron is added to an atom. |
| Example | Fluorine has the highest electronegativity value among all elements. | Chlorine has a higher electron affinity than fluorine. |
| Mathematical Representation | Electronegativity values are not directly calculable but are determined through experimental and theoretical methods. | Electron affinity values can be calculated using experimental techniques and theoretical models. |
| Chemical Behavior | Electronegativity influences how elements bond with each other to form compounds. | Electron affinity affects an atom’s tendency to gain electrons, which can impact its chemical reactivity and ability to form ions. |
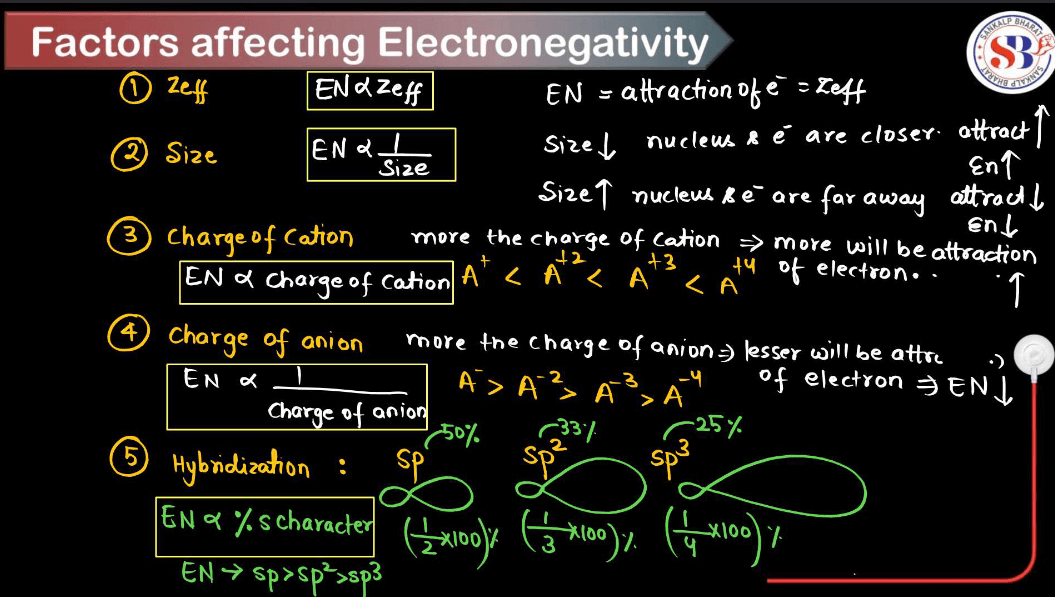
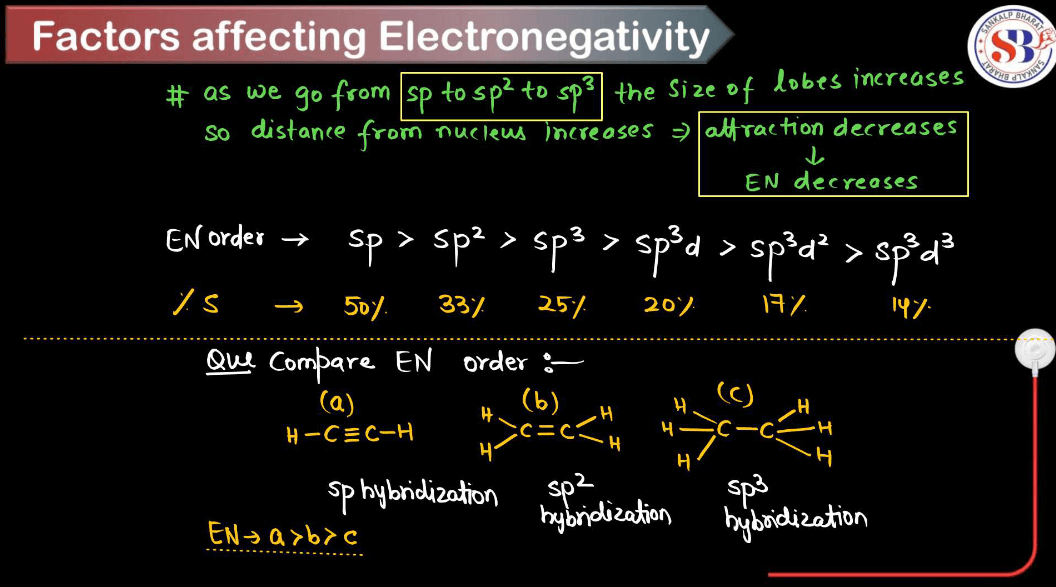
Factors Affecting Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. Several factors influence electronegativity. Understanding these factors helps predict the behavior of elements in chemical reactions and the types of bonds they form with other elements.
- Atomic Number: Electronegativity generally increases across a period (row) in the periodic table from left to right because as the atomic number increases, the number of protons in the nucleus increases, resulting in a stronger attraction for electrons.
- Distance from Nucleus: Electronegativity decreases down a group (column) in the periodic table because electrons are farther from the nucleus at higher energy levels, reducing the attractive force between the nucleus and outer electrons.
- Effective Nuclear Charge: This is the net positive charge experienced by valence electrons, which depends on the number of protons and the shielding effect of inner electrons. A higher effective nuclear charge increases electronegativity.
- Atomic Size: Generally, smaller atoms have higher electronegativity because the electrons are closer to the nucleus, experiencing a stronger attraction.
- Electron Configuration: The arrangement of electrons in an atom’s orbital influences electronegativity. Atoms with more electron shells have lower electronegativity due to increased shielding from inner electrons.
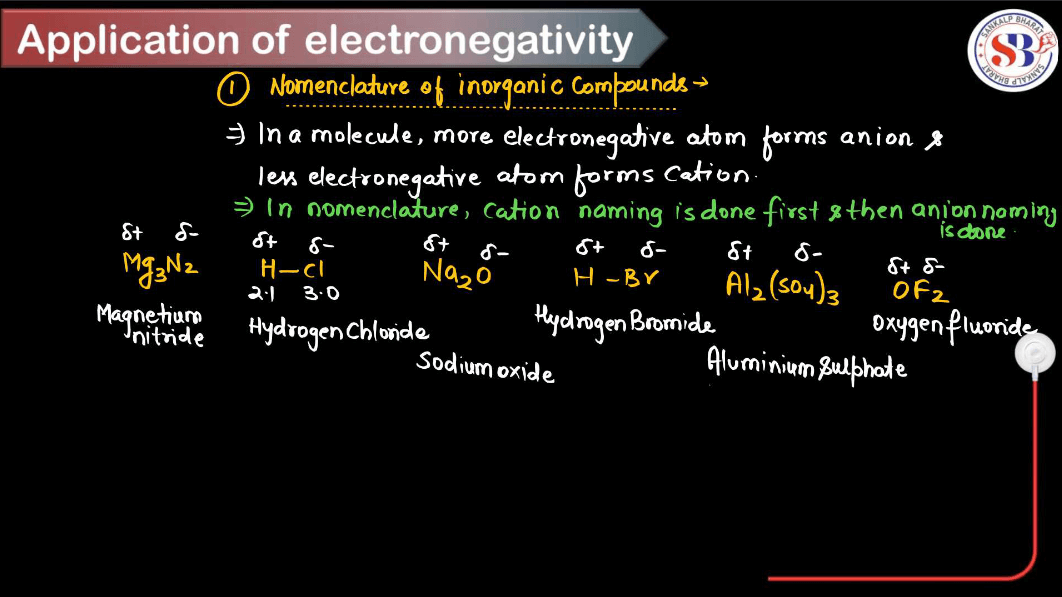
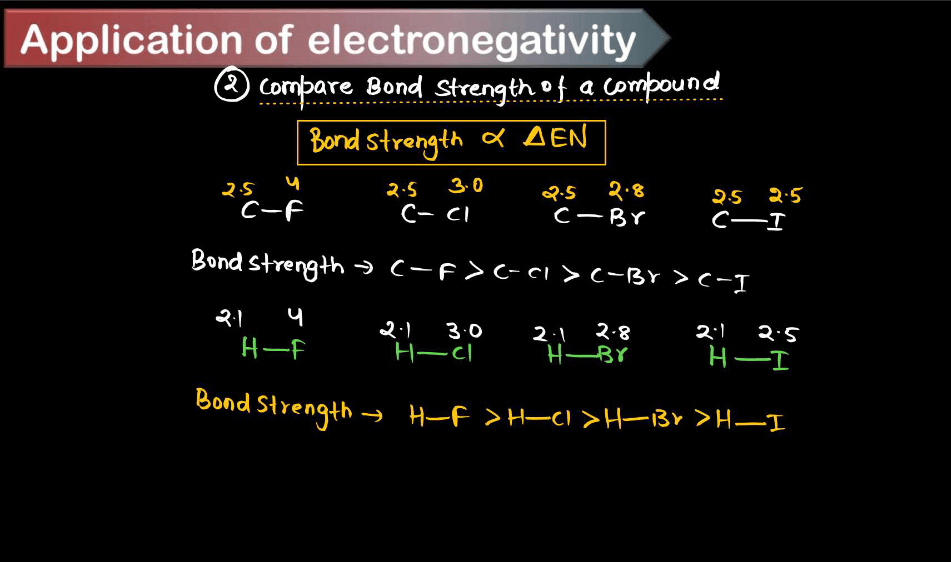
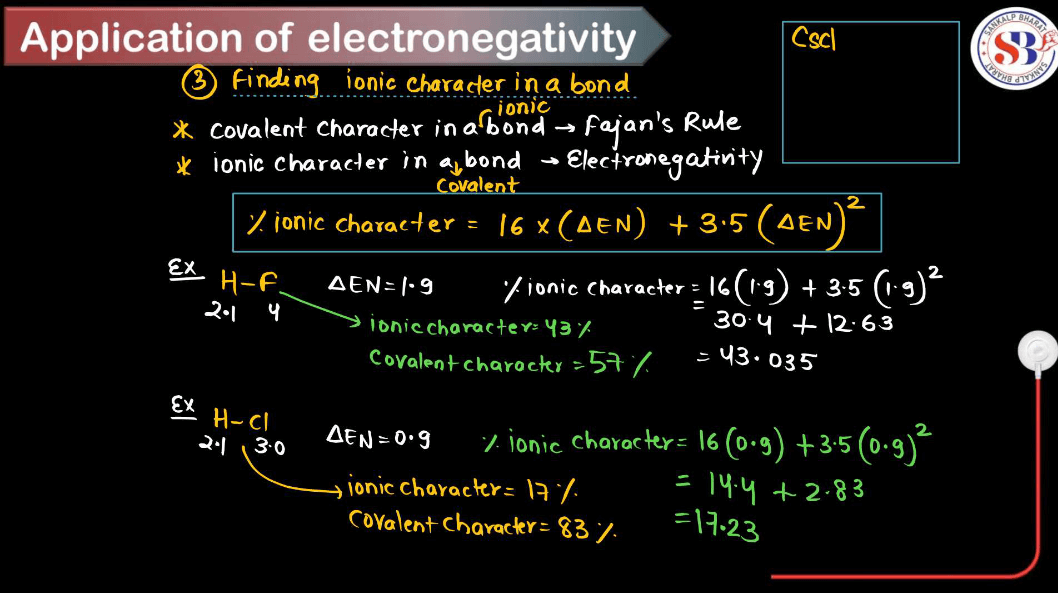
Applications of Electronegativity
Electronegativity is a fundamental concept in chemistry that measures the ability of an atom to attract electrons toward itself in a chemical bond. Here are some key applications of electronegativity. These applications highlight the importance of electronegativity in understanding chemical bonding, reactivity, and the properties of various substances in different contexts.
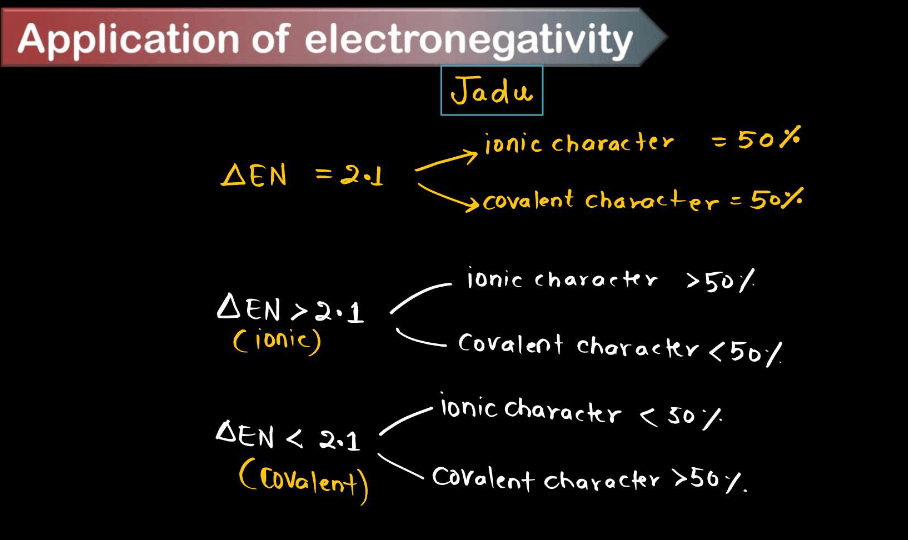
- Bond Type Prediction: Electronegativity differences between atoms can predict the type of bond formed between them. When the electronegativity difference is large (usually greater than 1.7), an ionic bond is formed; when the difference is moderate (around 0.5 to 1.7), a polar covalent is formed; and when the electronegativity difference is small (close to zero), a nonpolar covalent bond is formed.
- Chemical Reactivity: Electronegativity influences the reactivity of elements and compounds. Highly electronegative elements tend to be more reactive as they readily attract electrons, while less electronegative elements may be less reactive.
- Molecular Polarity: Electronegativity differences in molecules determine their polarity. Polar molecules have an uneven distribution of charge due to electronegativity differences between atoms, leading to the separation of positive and negative charges within the molecules.
- Solubility and Intermolecular Forces: Electronegativity plays a role in determining the types of intermolecular forces between molecules, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces. These forces affect dispersion forces. These forces affect properties like solubility, boiling point, and melting point.
- Acid-Base Properties: Electronegativity influences the acidity or basicity of compounds. For example, in the context of organic chemistry, the electronegativity of functional groups can affect the acidity of organic acids or the basicity of organic bases.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













