What is a Chemical Equation?
A chemical equation is like a recipe that describes a chemical reaction. It uses symbols and formulas to represent the substances involved and illustrates how they transform into new substances. On one side of the equation are the reactants – the ingredients going into the reaction. On the other side are the products – the outcomes of the reaction. The arrow in the middle signifies the direction of the reaction, showing the conversion from reactants to products.
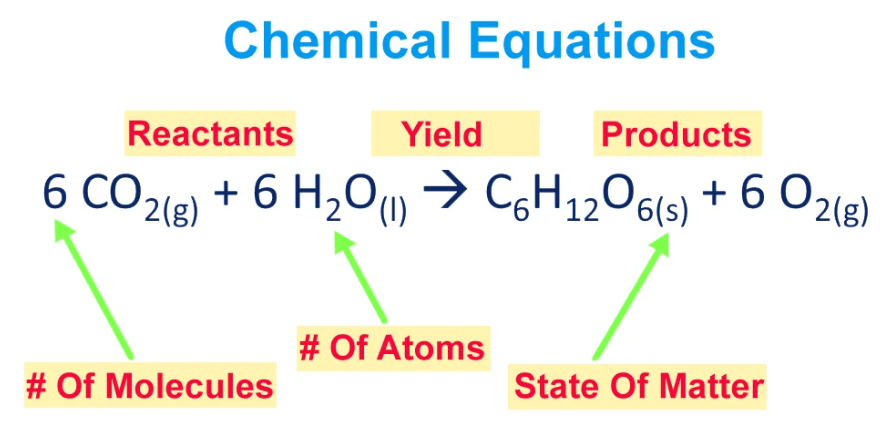
The numbers in front of the formulas, called coefficients, indicate the qualities of each substance involved, maintaining a balance between reactants and products. This balance follows the law of conservation of mass, stating that atoms are not created or destroyed in the chemical reaction, only rearranged. In simple terms, a chemical equation is a concise way of expressing the chemistry happening during a reaction, providing a snapshot of the molecular changes taking place.
Format of Writing a Chemical Equation
Writing a chemical equation involves representing a chemical reaction using symbols and formulas. Below we have discussed a step-wise format for writing a chemical equation.
Step I: Identify Reactants and Products: Determine the substances that are reacting (reactants) and the substances formed (products) in the chemical reaction.
Step II: Write Formulas: Use chemical symbols to write the formulas for each reactant and product. For example, H2O represents water, and CO2 represents carbon dioxide.
Step III: Balance the Equation: Ensure that the number of atoms of each element on the reactant side equals the number of atoms of the same element on the product side. Adjust coefficients (number in front of formulas) to achieve balance.
Step IV: Check State Symbols: Include state symbols (S for solid, I for liquid, G for gas, and aq for aqueous solution) after each substance to indicate its physical state during the reaction.
Step V: Include Reaction Conditions (Optional): Note any specific conditions required for the reaction, such as temperature, pressure, or the use of a catalyst.
Step VI: Double-Check for Accuracy: Review the entire equation to ensure it accurately represents the reaction, and all elements are balanced.
As an example, the formation of water from hydrogen and oxygen can be represented as:
2H2(g) + O2(g) → 2H2O(l)
This equation indicates that two molecules of hydrogen gas (H2) react with one molecule of oxygen gas (O2) to produce two molecules of water (H2O) in the liquid state.
Balancing of a Chemical Equation
Here’s a step-by-step method to balance a chemical equation. Remember to be patient and systematic, focusing on one element at a time, and you will balance the equation successfully.
Step I: Write the Unbalanced Equation: Write down the chemical equation with the correct chemical formula for the reactants and products.
Step II: Count the Atoms: Count the number of each type of atom on both sides of the equation.
Step III: Begin with the Most Complex Compound: Start balancing with the compound that has the most complex molecules or the one with the most types of atoms.
Step IV: Adjust Coefficients: Place coefficients in front of the compounds to balance the number of atoms. Avoid changing subscripts.
Step V: Balance Hydrogen and Oxygen Last: Typically, balance hydrogen and oxygen atoms last, as they often appear in multiple compounds.
Step VI: Check and Repeat: After adjusting coefficients, re-count the atoms on both sides. Repeat the process until the number of each type of atom is the same on both sides.
Step VI: Reduce to Simplest Whole Number: If necessary, divide all coefficients by the greatest common factor to ensure the coefficients are in the simplest whole-number ratio.
Define a Chemical Reaction
Chemical reactions are fundamental processes where substances, known as reactants, transform into different substances called products. At the molecular level, bonds between atoms are broken and new bonds are formed, resulting in a rearrangement of atoms to create distinct compounds. This transformation involves the conservation of reactant molecules into product molecules, often accompanied by changes in energy.
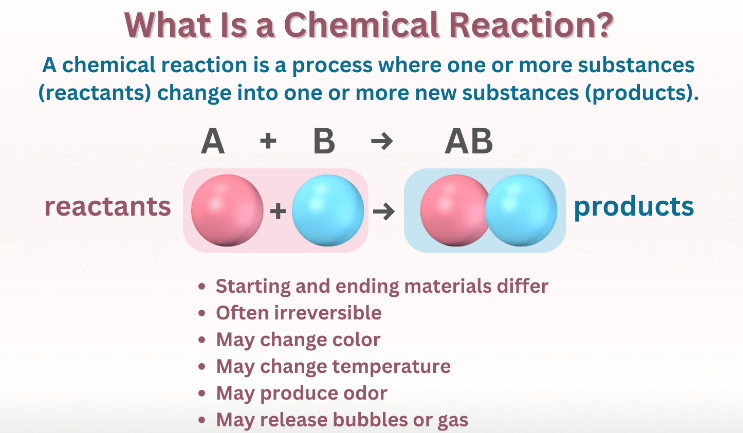
A simple example is the combination of hydrogen gas (H2) with oxygen gas (O2) to form water (H2O). In this reaction, bonds between hydrogen and oxygen atoms in the reactants are broken, and new bonds between hydrogen and oxygen atoms are created to yield water molecules. Importantly, the total number of atoms of each element remains constant; it’s just their arrangement that changes. Chemical reactions can be classified into various types, including synthesis (combining substances), decomposition (breaking down substances), and exchange reactions (swapping elements between molecules). These reactions play a crucial role in our daily life, influencing everything from Digestion to combustion in engines.
Types of Chemical Reactions
Chemical reactions can be classified into several types namely: Combination reactions, Decomposition reactions, Displacement reactions, Redox reactions, Precipitation reactions, and Polymerize reactions. These are fundamental categories, and many reactions may exhibit characteristics of more than one type.
| Different Types of Chemical Reactions | |
| Types of Reactions | Description |
| Combination reaction | In a combination reaction, two or more substances combine to form a new compound. Examples of combination reactions include A + B → AB. |
| Decomposition reaction | In a decomposition reaction, a single compound breaks down into simpler substances. Example includes: AB → A + B |
| Displacement reaction | In a displacement reaction, an element displaces another from a compound. Types include Single displacement and double displacement. |
| Redox reaction | Redox reaction involves the transfer of electrons between reactants. It includes Combustion reaction and Corrosion reaction. |
| Acid-Base reaction | It involves the combination of an acid and a base to form water and salt. Examples: HCl + NaOH → H2O + NaCl. |
| Precipitation reaction | Formation of an insoluble product when two solutions are product when two solutions are mixed. Examples include: AgNO3 + NaCl → AgCl + NaNO3 |
| Polymerization reaction | Small molecules (monomers) combine to form large molecules (polymers). Example include: nC2H4 → (C2H4)n. |
1. Combination reaction
A combination reaction is a chemical process where two or more substances combine to form a single new substance. This occurs when elements or compounds react to create a more complex product. For example, the combination of hydrogen and oxygen produces water: 2H2 + O2 → 2H2O. Similarly, the reaction between iron and sulfur results in iron sulfide: Fe + S → FeS. In these reactions, the reactants merge to create a distinct compound, showcasing the fundamental concept of combination reactions in Chemistry.
2. Decomposition reaction
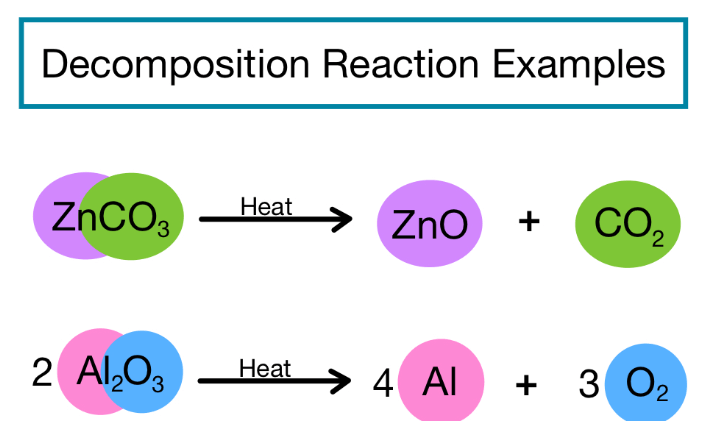
A decomposition reaction is a chemical transformation where a single substance breaks down into simpler components or elements. It’s the opposite of a combination reaction. An example is the decomposition of water into hydrogen and oxygen through electrolysis: 2H2O → 2H2 + O2. Another instance is the breakdown of hydrogen peroxide into water and oxygen: 2H2O2 → 2H2O + O2. These reactions highlight the disintegration of a complex substance into its constituent parts. Decomposition reactions play a crucial role in various natural and industrial processes, illustrating the diversity of chemical changes in the world around us.
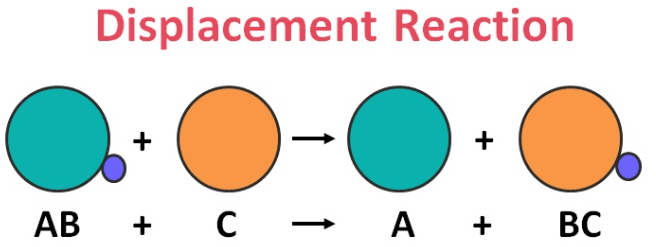
3. Displacement reaction
A displacement reaction, also known as a replacement or substitution reaction, occurs when one element replaces another in a compound. This usually involves a more reactive element displacing a less reactive one. For example, in the reaction between zinc and hydrochloric acid: Zn + 2HCL → ZnCl2 + H2, zinc displaces hydrogen from hydrochloric acid. Similarly, when copper reacts with silver nitrate: Cu + 2AgNO3 → Cu(NO3)2 +2Ag, copper displaces silver. Displacement reactions are essential in understanding the reactivity of elements and are commonly observed in various chemical processes, including the corrosion of metals and certain precipitation reactions.
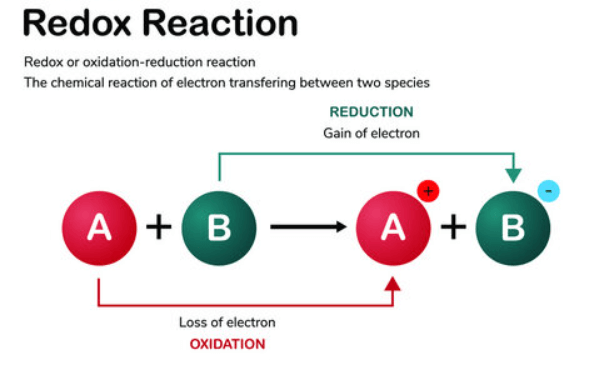
4. Redox Reaction
A redox (oxidation-reduction) reaction is a chemical process where one substance loses electrons (oxidation) while another gains electrons (reduction). The term “redox” combines these two reactions, as they always occur simultaneously. For instance, in the reaction between hydrogen and oxygen to form water: 2H2 + O2 → 2H2O, hydrogen is oxidized to release electrons, and oxygen is reduced by gaining these electrons. Another example is the reaction of iron with oxygen to form rust: 4Fe + 3O2 → 2Fe2O3. Redox reactions are fundamental in energy production, batteries, and various biological processes, showcasing electron transfer in chemical transformations.
Effects of Oxidation on Everyday Life
Oxidation reactions play a significant role in everyday life. Understanding oxidation reactions is crucial for various fields, from chemistry and Biology to materials science and environmental science. Here we have discussed a few examples of the effects of oxidation on everyday life.
- Rust Formation: The oxidation of iron in the presence of oxygen and water leads to the formation of rust. This process affects metal objects like cars, bridges, and tools, causing corrosion.
- Food Spoilage: Oxidation reactions contribute to the spoilage of food. For instance, when fruits, when fruits or vegetables are exposed to air, the oxygen promotes oxidation reactions that can lead to browning and degradation of nutrients.
- Respiration: In the human body, oxidation reactions are essential for cellular respiration. The process involves the oxidation of Glucose to produce energy, carbon dioxide, and water.
- Combustion: The burning of fuels, such as wood, gasoline, or natural gas, involves oxidation reactions. This process releases energy used for heating, cooking, and transportation.
- Battery Operation: Batteries rely on oxidation-reduction reactions to generate electrical energy. For example, in a common alkaline battery, zinc undergoes oxidation while manganese dioxide undergoes reduction.
To learn more about Chapter 1 Class 10 Science – Chemical Reactions and Equations, watch the video below by Sankalp Bharat Foundation:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













