A chemical bond is like a glue that holds atoms together to form molecules. Atoms together are like tiny building blocks of everything around us. When atoms come close, they can stick together through chemical bonds, which are like tiny hooks or magnets between them. These bonds can form when atoms share their outermost layer of electrons or when they give away or take electrons from each other. It’s kind of like a friendship between atoms, where they share or exchange things, to become stable. Different types of bonds, like covalent and ionic bonds, form in different ways depending on how atoms share or exchange electrons. These bonds give molecules their shape and properties.
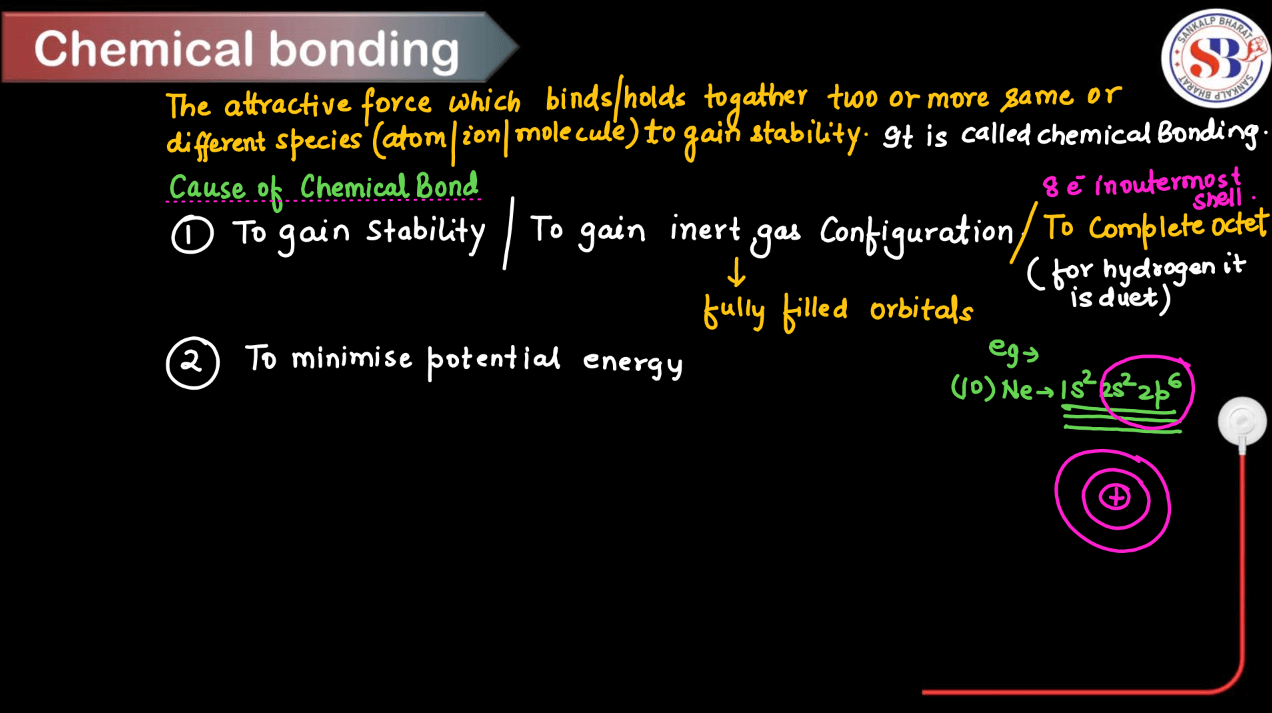
What is Chemical Bonding?
Chemical bonding is the way atoms join together to form molecules and compounds. Atoms are like puzzle pieces, and bonding is what connects them to create larger structures. Think of it as a kind of molecular glue. There are a few ways atoms can bond: they can share electrons, where they each contribute some electrons to form a strong connection called a covalent bond; they can give away or take electrons to form ions, which then attract each other through ionic bonds; or they can interact through weaker attractions called hydrogen bonds or van der Waals forces. The type of bonding between atoms determines the properties of the resulting molecules, like whether they’re solid, liquid, or gas, and how they interact with other substances.

Important Theories of Chemical Reaction
These theories provide different perspectives on how and why chemical reactions happen, helping scientists understand and manipulate them for various applications.
- Collision Theory: This theory suggests that for a chemical reaction to occur, reactant molecules must collide with enough energy and with the correct orientation. Think of it like two billiard balls needing to collide with enough force and in the right direction to pocket one another.
- Transition State Theory (Activated Complex Theory): It expands on collision theory, proposing that during a collision, reactant molecules form a high-energy intermediate state called the transition state or activated complex before forming the products. Imagine it like a roller coaster ride where the reactants need to go through a high point (transition state) before reaching the end (products).
- Arrhenius Equation: This equation relates the rate of a chemical reaction to temperature. It states that as temperature increases, the rate of reaction increases exponentially. In simpler terms, raising the temperature is like adding fuel to a fire, making the reaction go faster.
- Catalysis: Catalysis theory explains how substances called catalysts speed up reactions by providing an alternate pathway with a lower activation energy. It’s like a shortcut for reactants to reach the products more easily, facilitated by the catalyst.
- Chemical Kinetics: This area of study focuses on the speed or rate at which reactions occur and the factors that influence them, such as concentration, temperature, and catalysts. It’s like studying how fast cars move on a highway and the way factors affect their speed.
- Electron Transfer Theory (Redox Reaction): In redox reactions, one substance loses electrons (oxidation) while another gains them (reduction). This theory explains how electron transfer drives chemical changes, like how passing a baton in a relay race changes the runner’s position.
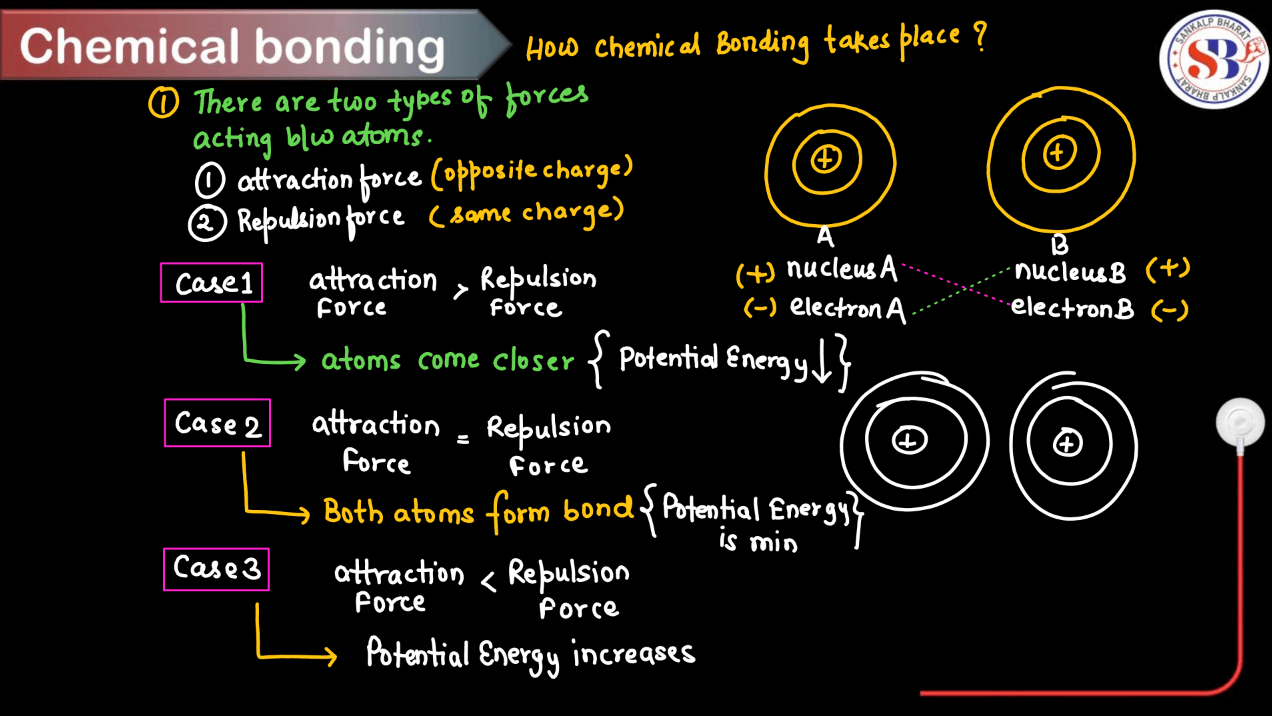
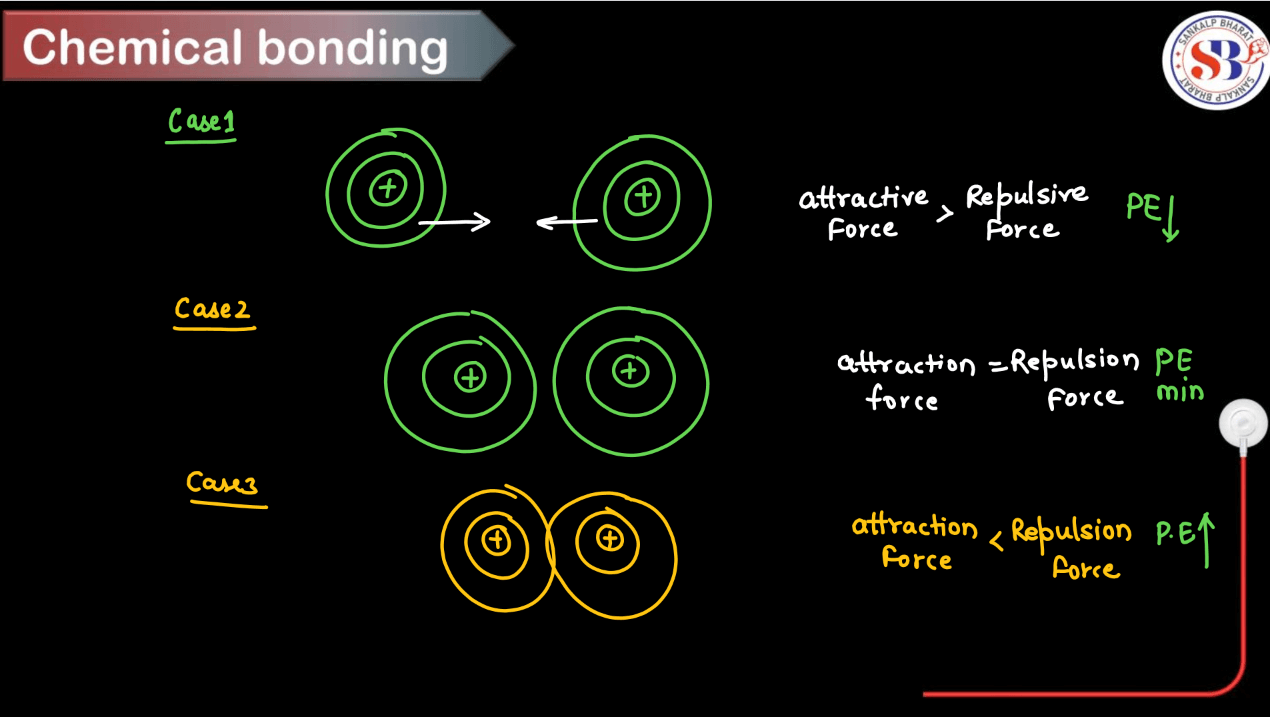
How Chemical Bonding Takes Place?
Chemical bonding occurs when atoms interact with each other to form compounds. By undergoing these steps, atoms achieve stable electron configurations, leading to the formation of various types of chemical bonds and compounds. Here’s a step-wise process of how chemical bonding takes place:
Step 1: Ionization or Electron Transfer: Atoms can gain or lose electrons to achieve a stable electron configuration (usually a full outer shell, like the nearest noble gas). This results in the formation of ions with positive or negative charges.
Step 2: Formation of Ionic Bonds: Oppositely charged ions are attached to each other due to electrostatic forces, forming ionic bonds. This is common in compounds composed of metals and nonmetals.
Step 3: Sharing of Electrons: Atoms can also share electrons to achieve stability. This sharing typically occurs between atoms of similar electronegativities and leads to the formation of covalent bonds.
Step 4: Formation of Covalent Bonds: Covalent bonds are formed when atoms share pairs of electrons. This sharing creates a stable electron configuration for both atoms involved.
Step 5: Polar Covalent Bonds: In some cases, atoms have different electronegativities, leading to unequal sharing of electrons. This results in polar covalent bonds, where one atom has a partial positive charge.
Step 6: Hydrogen Bond: Hydrogen bonds are a special type of dipole-dipole interaction where hydrogen atoms bonded to highly electronegative atoms (such as nitrogen, oxygen, or electronegative atoms).
Step 7: Van der Waals Forces: These are weak intermolecular forces that arise due to temporary dipoles induced in molecules. Van der Waals forces include London dispersion forces, dipole-dipole interaction, and hydrogen bonding.
Types of Chemical Bonding
Chemical Bonds can be broadly categorized into five main types including covalent bonds, Ionic Bonds, Hydrogen Bonds, Metallic Bonds, and Polar Bonds.
| Different Types of Chemical Bonding | |
| Types | Description |
| Covalent Bonds | Formed when atoms are electrons to achieve a stable electron configuration. Covalent bonds can be further classified into polar covalent bonds, where electrons are shared unequally, and nonpolar covalent bonds, where electrons are shared equally. Examples include the bonds between hydrogen and oxygen in water (H2O). |
| Ionic Bonds | Formed when one atom transfers electrons to another atom, resulting in the formation of positively and negatively charged ions that are attracted to each other. Examples include the bond between sodium and chlorine in table salt (NaCl). |
| Hydrogen Bonds | These bonds are formed between a hydrogen atom bonded to a highly electronegative atom such as nitrogen, oxygen, or fluorine and another electronegative atom in a different molecule. Hydrogen bonds are weaker than covalent bonds but still important in determining the structure and properties of molecules. An example is the hydrogen bonding between water molecules. |
| Metallic Bonds | Occur between atoms of metallic elements and involve the sharing of many electrons among a lattice of atoms. This results in a “sea” of delocalized electrons that can move freely throughout the material, giving metals their characteristic properties such as conductivity and malleability. Eg. include the bonds within a piece of solid metal like iron or copper. |
| Polar Bonds | Polar bonds occur when there is an unequal sharing of electrons between atoms due to differences in electronegativity. This results in a partial positive charge on one atom and a partial negative charge on the other. Polar bonds can exist within molecules (intramolecular) or between molecules (intermolecular). |
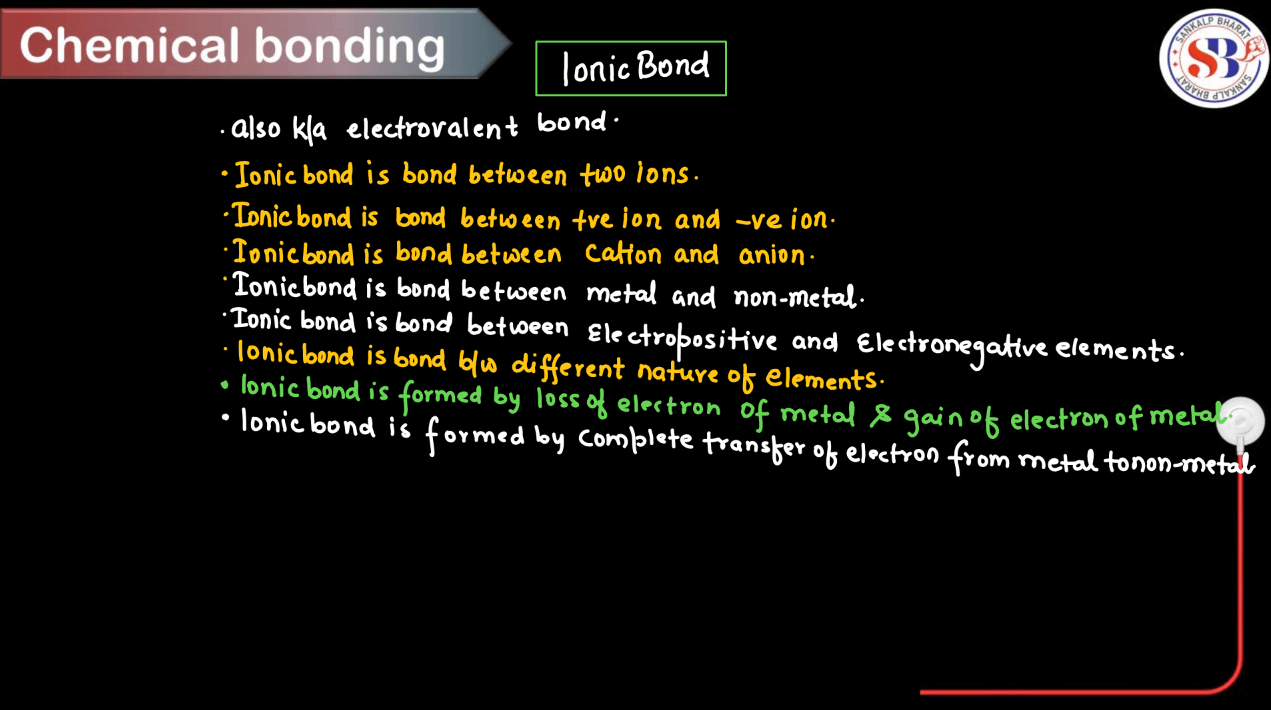
Ionic Bonds
Ionic bonds are like magnets attracting oppositely charged ions. When atoms with different electronegativities meet, one atom tasks electrons from the other, creating positive and negative ions. Imagine one atom happily giving away its electron to another, creating an electrically charged duo. These ions are drawn together by their opposite charges, forming a strong bond. It is like the attraction between the positive and negative ends of magnets. Ionic bonds are common in salts like sodium chloride (table salt) and play a crucial role in forming compounds in chemistry, such as in the structure of crystals and minerals.
Covalent Bonds
Covalent bonds are like friendly handshakes between atoms. Atoms share electrons to complete their outer shells, forming a strong bond. Imagine two friends holding hands, each sharing the responsibility of keeping the other stable. In covalent bonds, atoms share electrons equally or unequally, depending on their electronegativity. When atoms have similar electronegativity, they share electrons equally, forming a nonpolar covalent bond. If one atom has higher electronegativity, it pulls the shared electrons closer, forming a polar covalent bond. These bonds hold molecules together, creating compounds essential for life, like water and DNA.
Theories of Covalent Bonds
There are several theories that explain the nature of covalent bonds. Each of these theories provides a different perspective on the nature of covalent bonds, and they are often used in combination to understand molecular structures and properties.
- Valence Bond Theory (VBT): This theory suggests that a covalent bond forms when two atomic orbitals overlap and share electrons. It emphasizes the idea of electron pairing between atoms.
- Molecular Orbital Theory (MO Theory): MO theory considers the entire molecule’s electronic structure by forming molecular orbitals from the combination of atomic orbitals. It describes bonding as the result of constructive interference of atomic orbitals.
- Resonance: This theory suggests that in some molecules, the bonding cannot be accurately represented by a single Lewis structure. Instead, it is described as a resonance hybrid of multiple structures.
- VSEPR Theory (Valence Shell Electron Pair Repulsion Theory): While not strictly a theory of bonding, VSEPR theory predicts the shapes of molecules based on the idea that electron pairs around a central atom repel each other, leading to a geometry that minimizes repulsion.
- Lewis Dot Theory: This theory, proposed by Gilbert N. Lewis, emphasizes the formation of covalent bonds. It represents atoms as symbols surrounded by dots representing valence electrons, and it helps predict the number and types of bonds an atom can form.
- Hybridization Theory: Hybridization theory explains the mixing of atomic orbitals to form hybrid orbitals, which have different shapes and energies from the orbital atomic orbitals. This theory helps explain the geometry and bonding in molecules, especially in cases where simple atomic orbitals cannot fully explain molecular shapes, like in methane (CH4) where carbon undergoes sp3 hybridization.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













