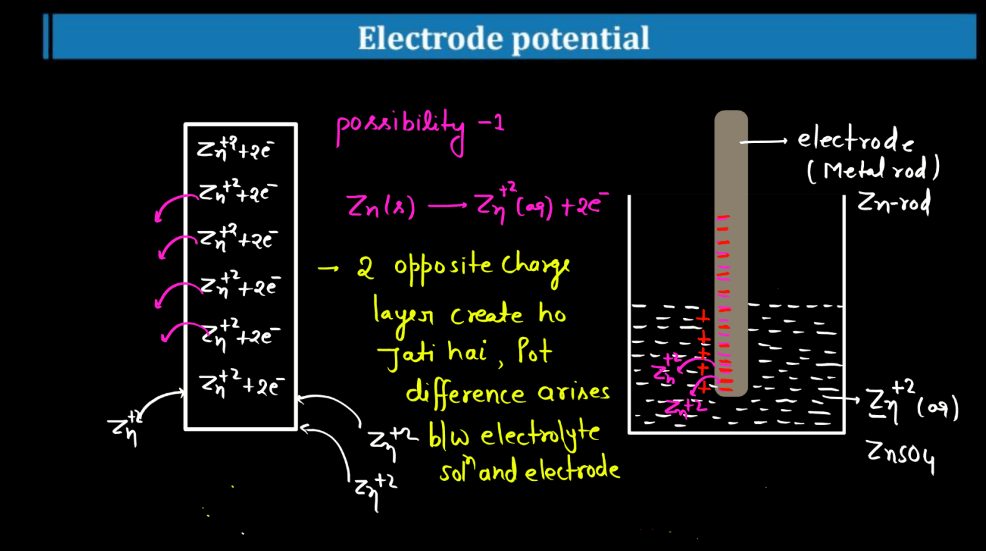
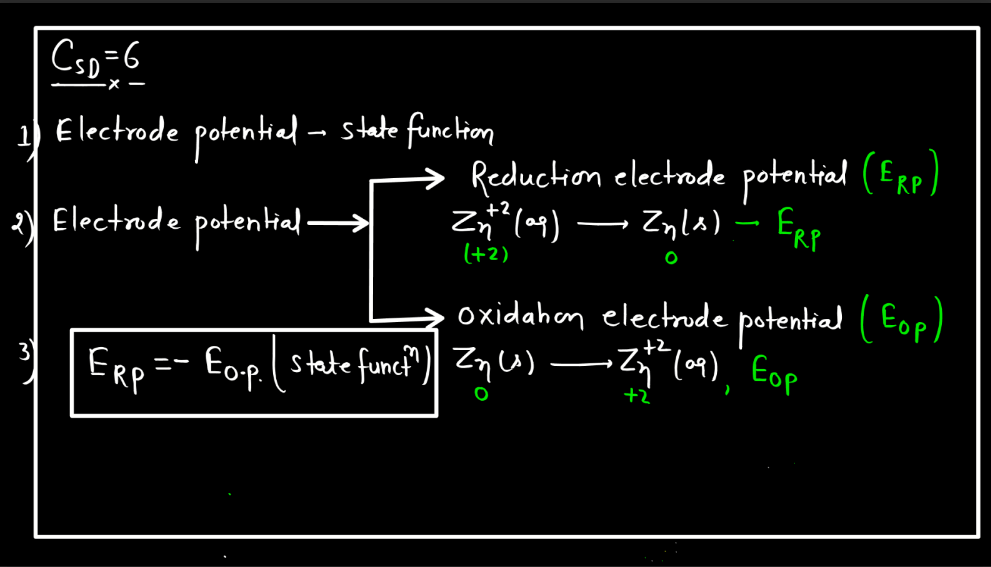
Electrode Potential
Electrode potential is a measure of how eager an electrode (a conductor that allows the flow of electric current) is to either gain or lose electrons during a chemical reaction. It indicates the tendency of a substance to be oxidized (lose electrons) or reduced (gain electrons) at an electrode. In simple terms, think of it as the eagerness of a material to give away or accept electrons when it participates in a reaction. A positive electrode potential suggests a willingness to lose electrons (oxidation), while a negative electrode potential indicates a preference for gaining electrode potential in volts (V), and it helps us understand the direction and strength of the electrochemical reactions happening in batteries, cells, and various chemical processes.
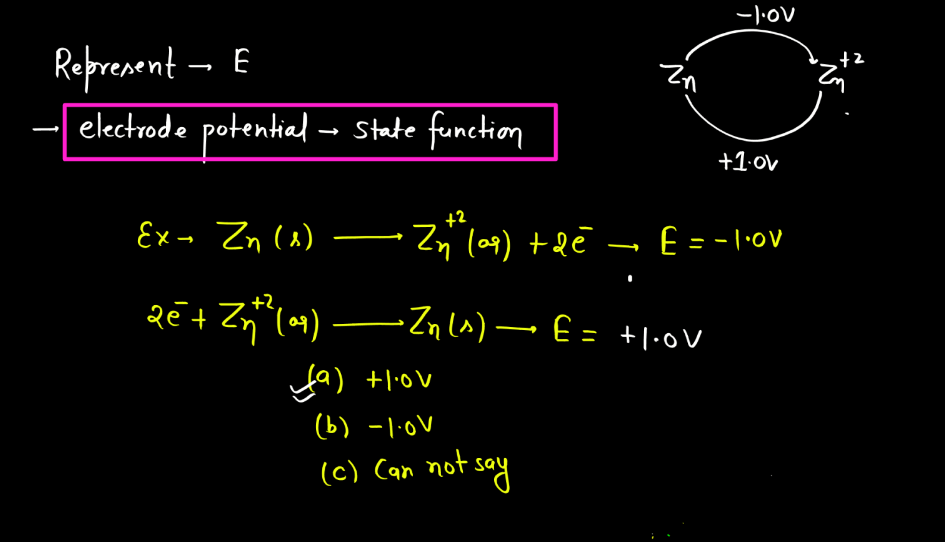
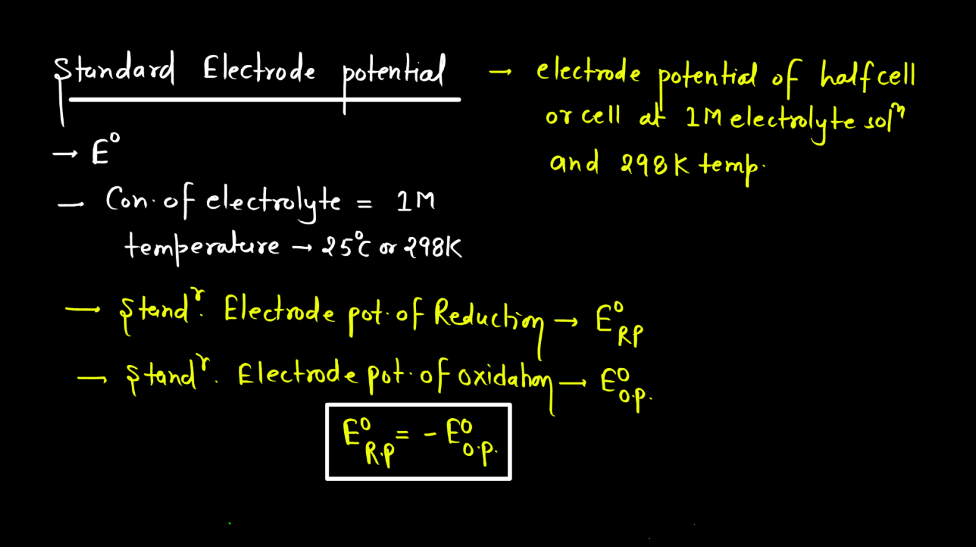
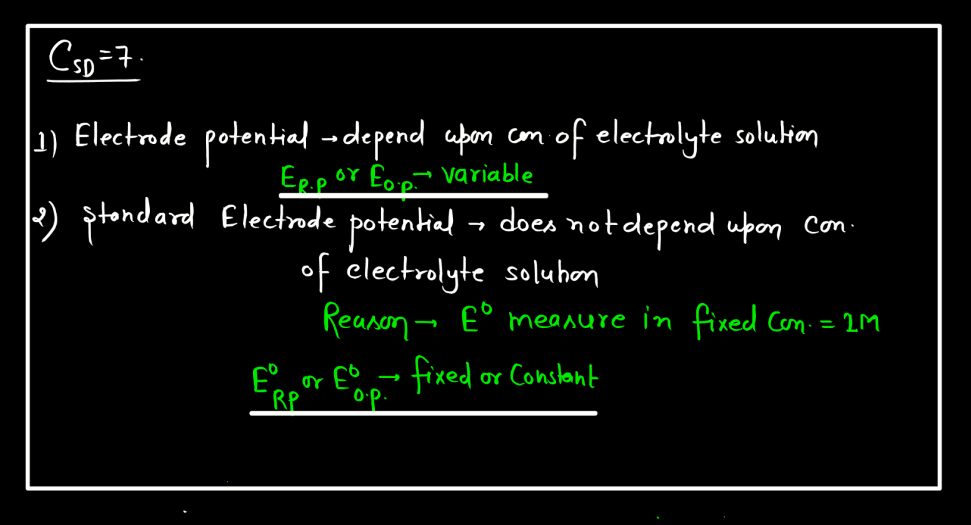
Standard Electrode Potential
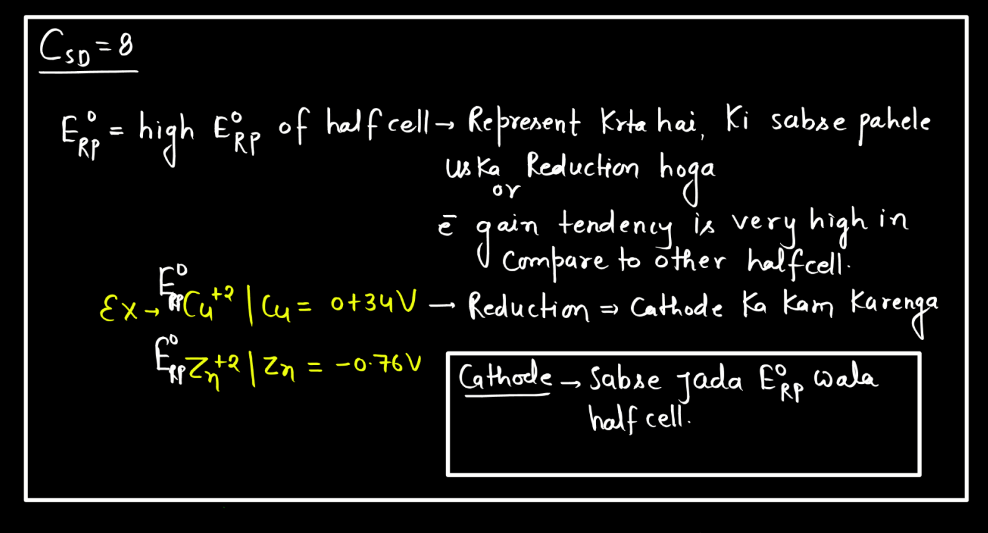
Standard electrode potential is a measure of how strongly an electrode wants to either gain or lose electrons compared to a standard reference electrode. This reference is the hydrogen electrode under standard conditions. Each substance has its own standard electrode potential, indicating its tendency to undergo oxidation or reduction. A higher standard electrode potential means the substance is more likely to gain electrons and be reduced, while a lower potential suggests a greater tendency to lose electrons and undergo oxidation.
This measurement helps predict the direction of electron flow in electrochemical reactions and provides insights into the feasibility of these reactions. Essentially, it’s like a scoreboard for the eagerness of substances to participate in chemical changes involving electron transfer, providing a standardized way to compare their reactivity in electrochemical processes.
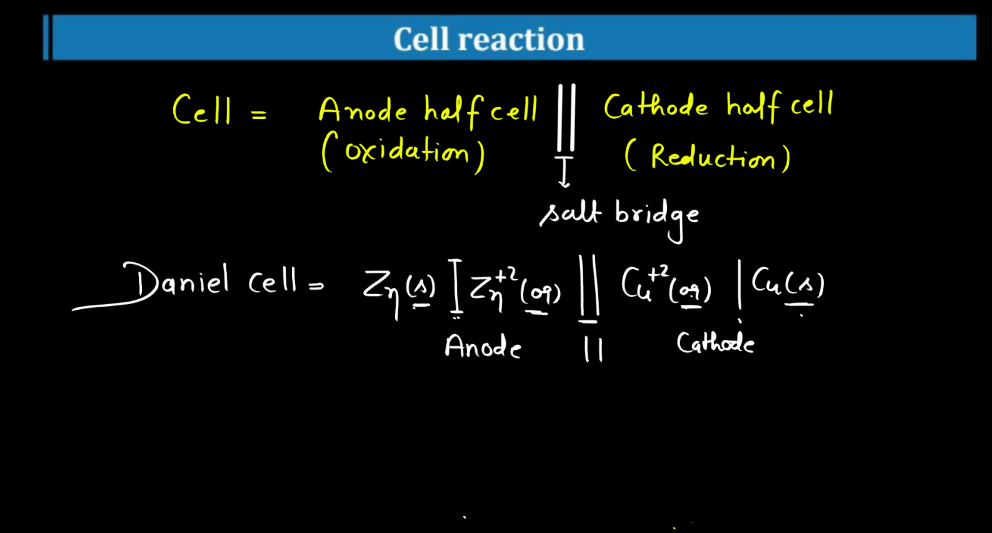
Cell Reaction
A cell reaction involves the conservation of chemical substances into different forms during electrochemical processes. In simple language, let’s consider a common example: the reaction that occurs in a simple battery. In a typical alkaline battery, zinc undergoes a reaction with manganese dioxide. The zinc atoms lose electrons, becoming positively charged ions (Zn^2+), while manganese dioxide gains those electrons, transforming into ions with a low positive charge (Mn^3+). This electron transfer creates an electric current that powers devices connected to the battery. The overall reaction can be represented as:
Zn(s) + 2MnO2(s) + 2H2O(l) → Zn(OH)2(s) + 2Mn(OH)2(s)
In simple terms, during this chemical process, zinc gives away electrons to manganese dioxide, generating electrical energy in the form of a current.
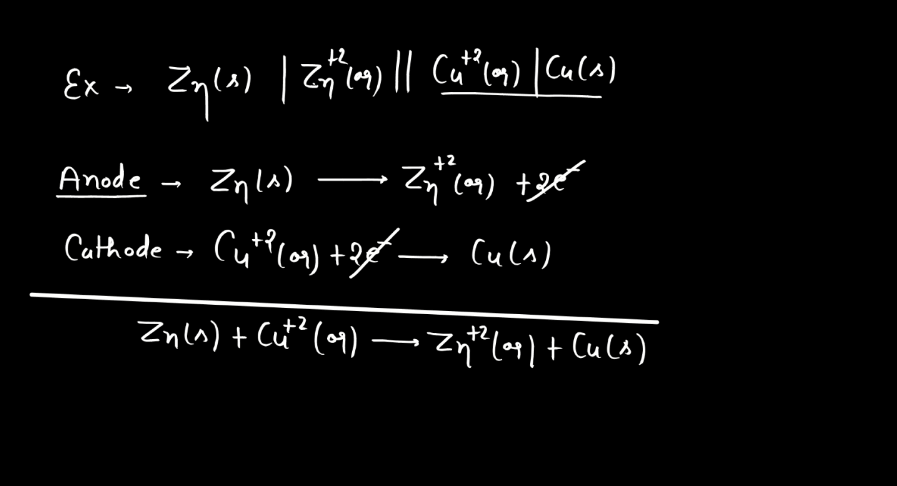
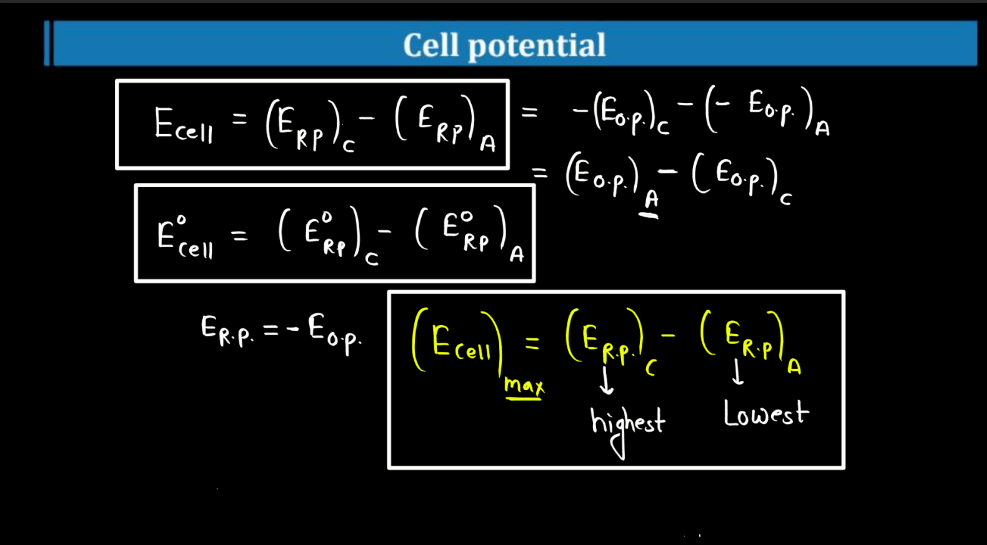
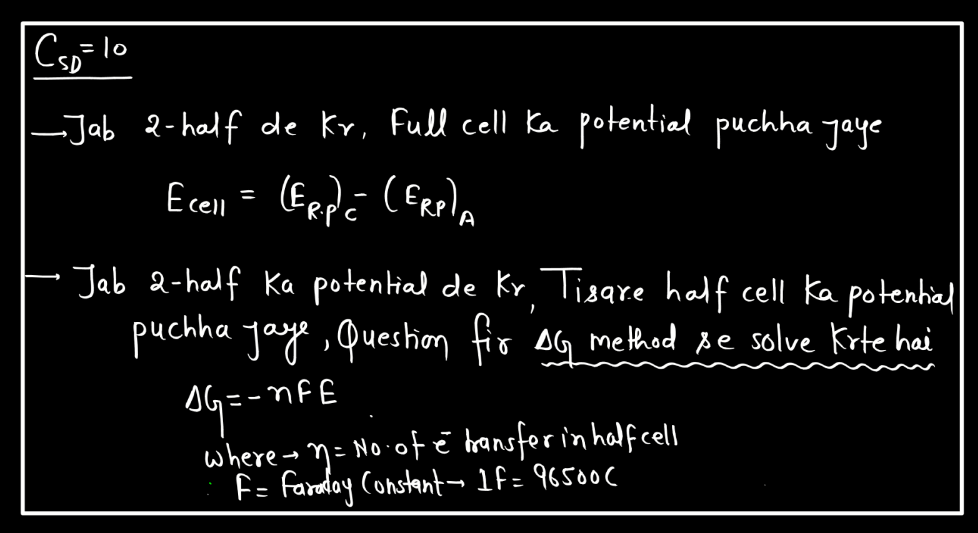
Cell Potential
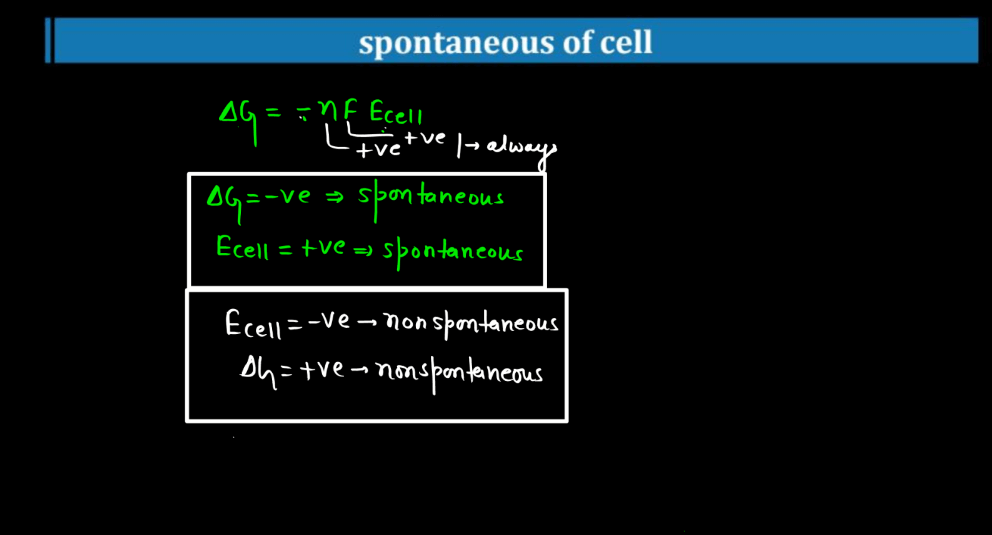
Cell potential, also known as voltage or electromotive force (EMF), measures the driving forces for electrons to flow in an electrochemical cell. Imagine it as the energy available to push electrons through a circuit. It arises from the chemical reactions occurring in the cell. Each half-cell (oxidation and reduction) contributes to the overall potential. In simple terms, it’s like the water pressure in a hose – the higher the pressure, the more water flows. Similarly, the higher the cell potential, the more electrons move through the circuit. If the cell potential is positive, the reaction is spontaneous and can produce electrical energy. Conversely, a negative cell potential indicates a non-spontaneous reaction. Cell potential is crucial in understanding and designing batteries and other electrochemical systems.
Spontaneous of Cell
Spontaneous cell generation, often referred to as spontaneous generation, is a process where living organisms seemingly appear from non-living matter without any apparent cause or external influence. This concept was widely accepted in the past, but scientific advancements, particularly the work of Louis Pasteur in the 19th century, debunked the idea. Pasteur demonstrated through experiments that life does not spontaneously arise from inanimate substances. Instead, living organisms come from pre-existing living cells. This understanding laid the foundation for the principle of biogenesis, emphasizing that life originates from other living entities. Spontaneous cell generation has been discredited, paving the way for a more accurate comprehension of the intricate processes underlying life’s emergence and continuity.
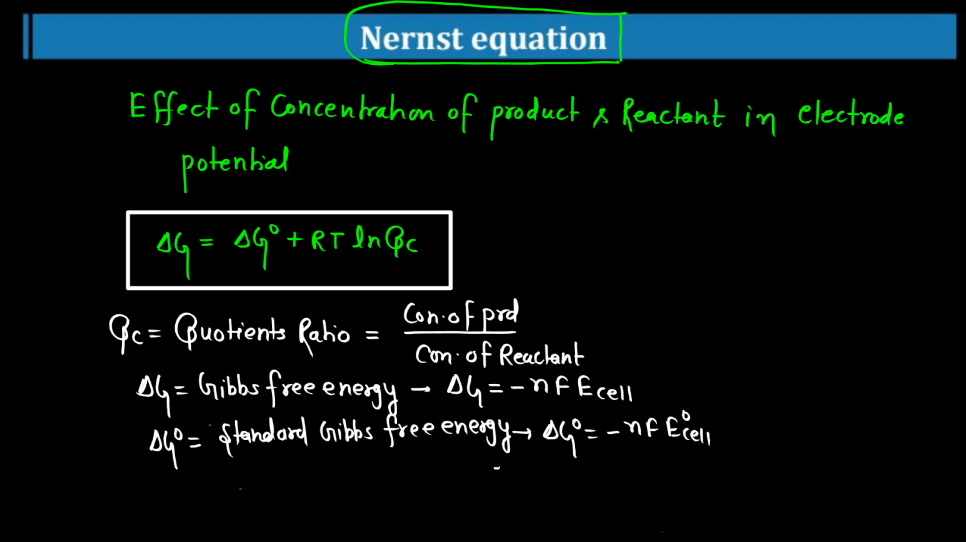
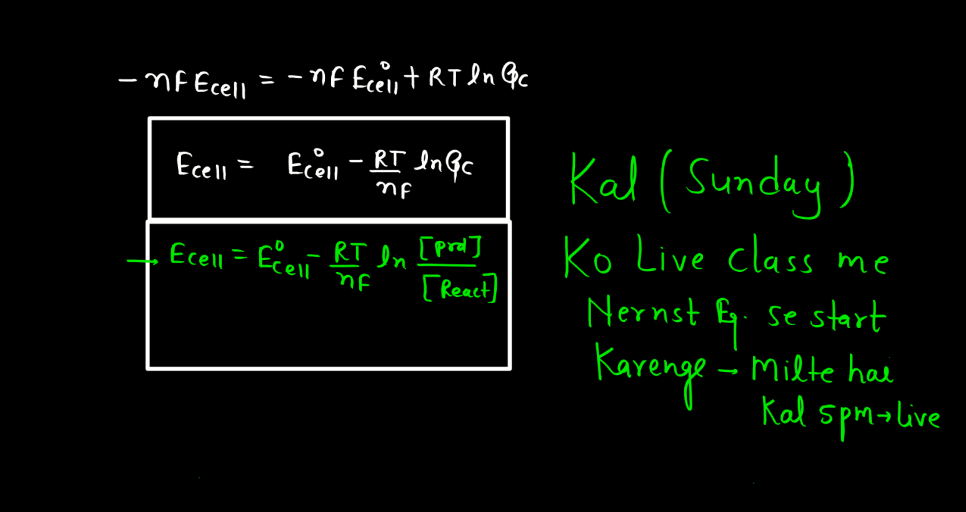
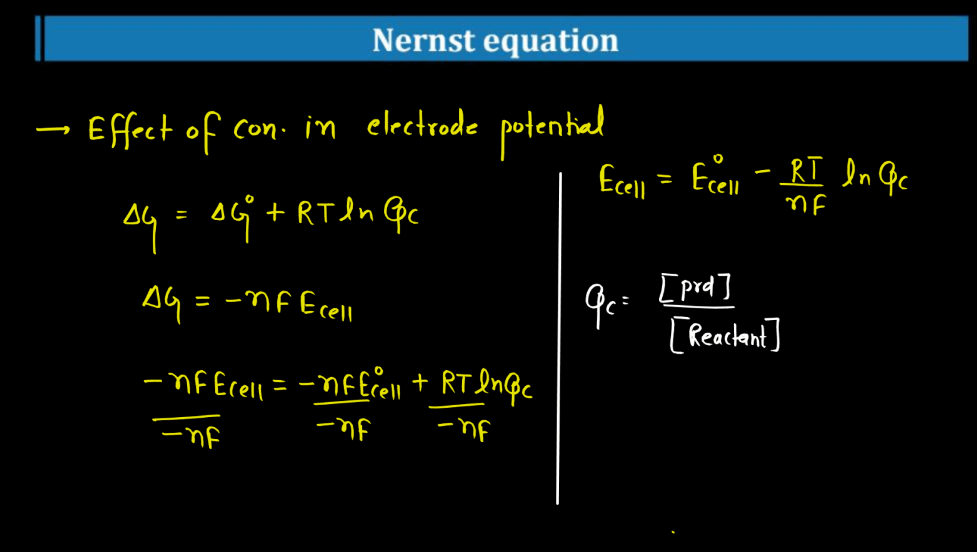
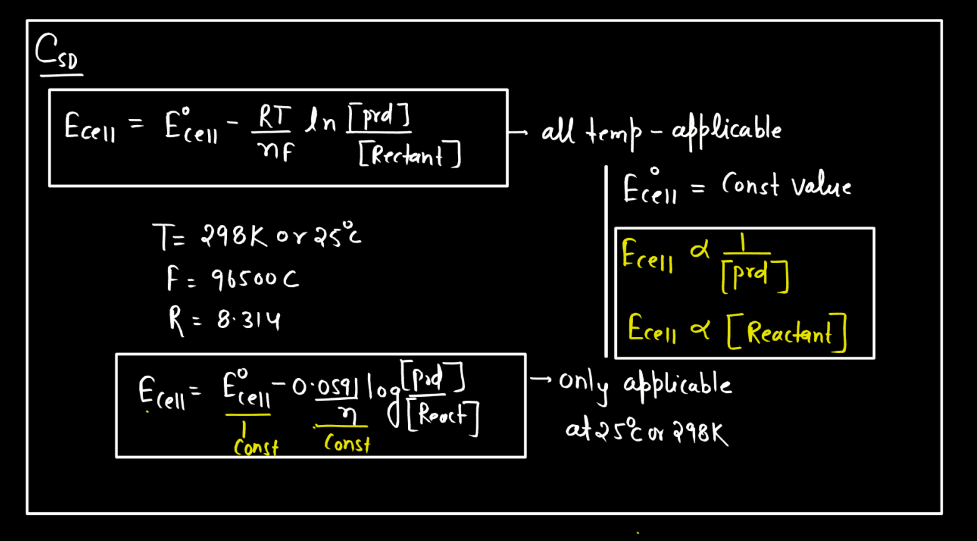
Nernst Equation
The Nernst Equation is a mathematical expression that relates the concentration of ions on either side of a cell membrane to the cell’s electrical potential. Named after the German physicist Walther Nernst, this equation helps describe the electrochemical behavior of cells, like neurons. In simple terms, it tells us how the distribution of ions (charged particles) influences the electrical charge across a cell membrane. The equation involves the concentrations of ions inside and outside the cell, as well as a constant and the temperature.
Essentially, it explains how the movement of ions across the membrane can generate an electrical voltage, crucial for various biological processes. This equation is particularly significant in neuroscience, where it helps us understand how nerve cells transmit signals. By examining the Nernst Equation, scientists can gain insights into the mechanisms underlying the electrical activity of cells and how they communicate in complex systems like the Human Brain.
For more information, watch the complete video given below:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













