Atomic mass in chemistry refers to the weight of an atom, mainly determined by the number of protons and neutrons in its nucleus. Protons and neutrons each have a mass of about 1 atomic mass unit (amu), so the atomic mass is roughly the sum of these particles. Electrons are much lighter and don’t significantly affect the atomic mass. Atomic mass helps us understand the weight and composition of different elements in the periodic table. For more information regarding the Atomic Mass of Elements From 1 to 30 go through the complete article below.
What is Atomic Mass?
Atomic mass is the weight of an atom, which is the smallest part of an element. It tells us how heavy the atom is. This mass is mostly made up of protons and neutrons found in the nucleus (center) of the atom. Electrons, which are much smaller, also exist in the atom but don’t add much to its mass. Each proton and neutron is given a mass of about 1 atomic mass unit (amu). So, the atomic mass is often close to the total number of protons and neutrons in an atom. For example, a carbon atom, which has 6 protons and 6 neutrons, has an atomic mass of about 12 amu. A helium atom has 2 protons and 2 neutrons, giving it an atomic mass of about 4 amu.
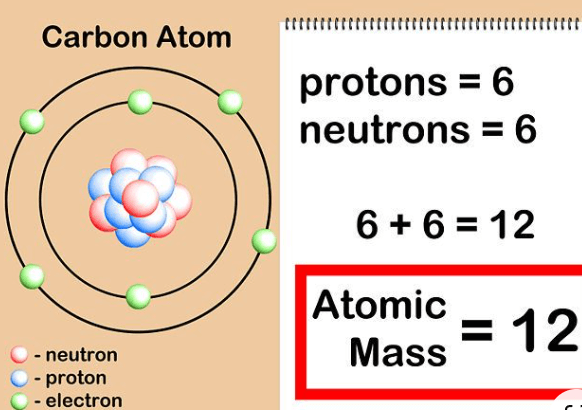
Or in more simpler terms, We can say “Atomic mass is the weight of an atom, mostly from its protons and neutrons. It measures how heavy the atom is and is usually close to the total number of these particles in the atom’s nucleus.”
Atomic Mass of Elements From 1 to 30
The atomic mass of an element is the average mass of its atoms, measured in atomic mass units (amu). For elements 1 to 30, the atomic mass starts small and increases as you move to higher numbers. For example, hydrogen (element 1) has an atomic mass of about 1 amu, while calcium (element 20) has an atomic mass of about 40 amu. The trend is that as elements get heavier, their atomic masses increase. This is because each atom has more protons and neutrons in its nucleus, making it heavier. The table mentioned below shows the atomic mass and atomic number of the top 30 elements.
| Table of First 30 Elements with Atomic Masses | ||
| Atomic Number | Elements with Symbols | Atomic Mass |
| 1 | Hydrogen (H) | 1.008 |
| 2 | Helium (He) | 4.0026 |
| 3 | Lithium (Li) | 6.94 |
| 4 | Beryllium (Be) | 9.0122 |
| 5 | Boron (B) | 10.81 |
| 6 | Carbon (C) | 12.011 |
| 7 | Nitrogen (N) | 14.007 |
| 8 | Oxygen (O) | 15.999 |
| 9 | Fluorine (F) | 18.998 |
| 10 | Neon (Ne) | 20.180 |
| 11 | Sodium (Na) | 22.990 |
| 12 | Magnesium (Mg) | 24.305 |
| 13 | Aluminium (Al) | 26.982 |
| 14 | Silicon (Si) | 28.085 |
| 15 | Phosphorus (P) | 30.974 |
| 16 | Sulfur (S) | 32.06 |
| 17 | Chlorine (Cl) | 35.45 |
| 18 | Argon (Ar) | 39.948 |
| 19 | Potassium (K) | 39.098 |
| 20 | Calcium (Ca) | 40.078 |
| 21 | Scandium (Sc) | 44.956 |
| 22 | Titanium (Ti) | 47.867 |
| 23 | Vanadium (V) | 50.942 |
| 24 | Chromium (Cr) | 51.996 |
| 25 | Manganese (Mn) | 54.938 |
| 26 | Iron (Fe) | 55.845 |
| 27 | Cobalt (Co) | 58.933 |
| 28 | Nickel (Ni) | 58.693 |
| 29 | Copper (Cu) | 63.546 |
| 30 | Zinc (Zn) | 65.38 |
Atomic Masses of 1 to 30 Elements with Symbols PDF
Calculation of Atomic Mass of Elements
The atomic mass of an element is the average mass of all the atoms of that element. Atoms are made of protons, neutrons, and electrons. The atomic mass mostly comes from protons and neutrons because electrons are very light. Each proton and neutron is counted as one unit of mass. To find the atomic mass, scientists add up the number of protons and neutrons in the atom’s nucleus.
Since most elements have different forms (called isotopes), the atomic mass is an average that takes into account the different amounts of each isotope. This average is why the atomic mass often isn’t a whole number. For example, the atomic mass of carbon is 12.01 because it’s an average of all the different carbon atoms.
The formula for calculating the Atomic Mass of an element is:
| Atomic Mass Number = Mass of Protons + Mass of Neutrons + Mass of Electrons |
Step-Wise Process for Calculating Atomic Mass of Elements
Step 1: Identify Isotopes:
Find out the different isotopes of the element. Isotopes are atoms of the same element that have different numbers of neutrons.
Step 2: Find the Mass of Each Isotope:
Determine the mass of each isotope. This is usually given in atomic mass units (amu).
Step 3: Determine Abundance:
Find the percentage abundance of each isotope. This tells you how common each isotope is in nature.
Step 4: Multiply Mass by Abundance:
Multiply the mass of each isotope by its percentage abundance (expressed as a decimal).
Step 5: Add the Products:
Add up all the values from the previous step. The sum is the atomic mass of the element.
Is Atomic Number and Atomic Mass different?
The atomic number is a number that tells us how many protons are in the nucleus of an atom. Each element on the periodic table has a unique atomic number. For example, hydrogen has an atomic number of 1 because it has one proton. Oxygen has an atomic number of 8 because it has eight protons. This number helps determine the identity of the element and its position on the periodic table. It also tells us about the element’s chemical properties and how it will react with other elements. So, the atomic number is like a special code that identifies each type of atom and helps us understand more about its behavior. The table mentioned below highlights the key differences between atomic mass and atomic number:
| Difference Between Atomic Number and Atomic Mass | ||
| Aspect | Atomic Mass | Atomic Number |
| Definition | The weighted average mass of an atomic mass of an atomic’s isotopes. | The number of protons in an atom’s nucleus. |
| Symbol | Often represented as ‘A’ or ‘M’ in equations | Represented as ‘Z’ in equations. |
| Measurement Unit | Measured in atomic mass unit [amu]. | Unitless. |
| Determines | Determines the mass of the atom. | Determines the element and its position on the periodic table. |
| Calculation | Calculated using the masses of isotopes and their relative abundances. | Directly equal to the number of protons. |
| Variability | Varies between isotopes of the same element. | Constant for all atoms of a given element. |
| Use | Used to calculate the mass of atoms and molecules. | Used to identify and classify elements. |
| Position on Table | Not directly shown on the periodic table. | Shown on the periodic table as the element’s position. |


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













