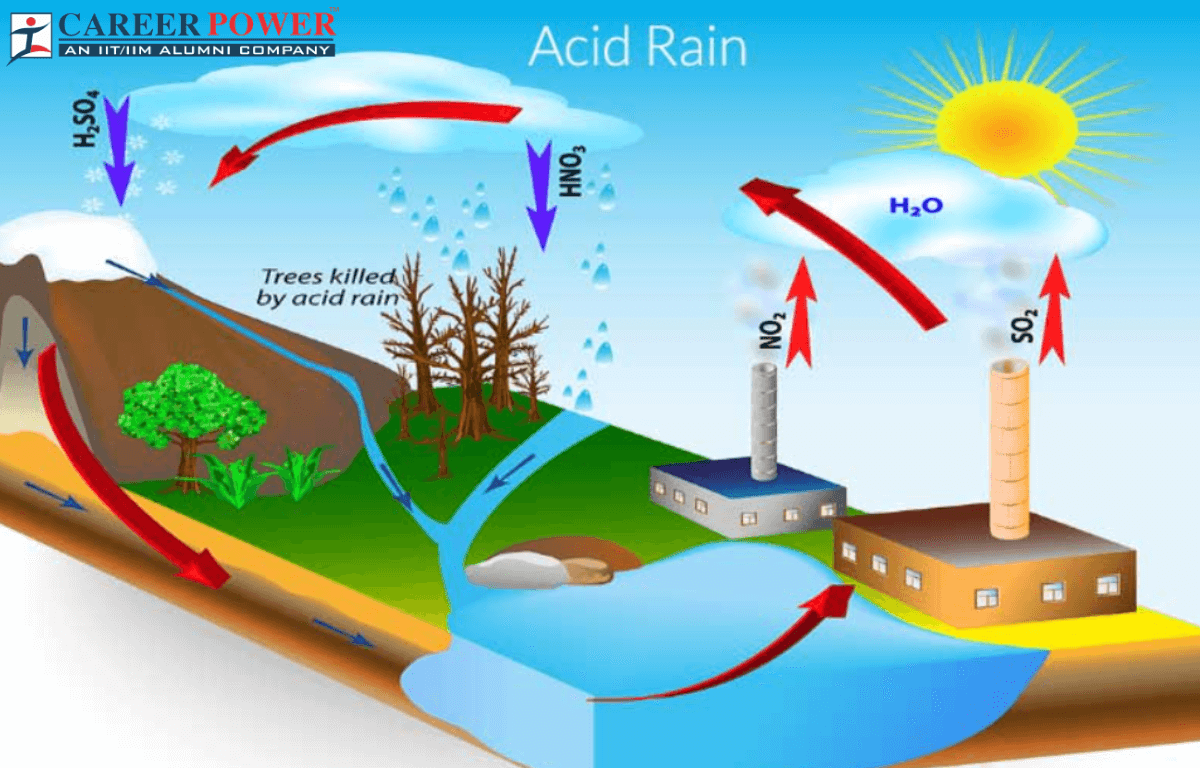
Acid rain, a byproduct of industrialization and the burning of fossil fuels has long been recognized as a significant environmental issue. It is not a natural phenomenon; we can say that acid rain is a result of human activities. The pollutants released at the time of these activities combine with atmospheric moisture to form sulfuric acid and nitric acid, which fall to the Earth’s surface in rain, snow, fog, or dust. These acidic compounds lower the pH of precipitation, leading to the term “acid rain”. Acid rain is also an important topic in biology.
Acid Rain Definition
Acid rain is a type of environmental pollution caused by emissions of sulfur dioxide (SO2) and nitrogen oxide (NOx) from sources like industrial processes and vehicle emissions. These gases react with water vapor and other chemicals in the atmosphere to form acidic compounds, which can fall to the Earth’s surface as rain, snow, or other forms of precipitation. Acid rain can harm the ecosystem, damage buildings and infrastructures, and have negative health effects on humans and wildlife. Efforts to reduce emissions of sulfur dioxide and nitrogen oxides have been made to mitigate its impact.

Examples of Acid Rain Incidents
Let us discuss some of the real-life examples of acid rain incidents and their effects:
- Statue of Liberty (New York, USA): The iconic Statue of Liberty faced corrosion due to extensive restoration work in the 1980s.

- Northeastern United States and Canada (1970s-1980s): This region experienced severe acid rain problems due to emissions from industrial activities and power plants. Acid rain caused significant damage to forests and aquatic ecosystems, leading to government regulations to reduce emissions.
- Scandinavian Lakes and Forests: Sweden and Norway suffered from acidified lakes and damaged forests. These countries have undertaken substantial mitigation efforts and have seen some recovery in affected ecosystems.
- Taj Mahal, India: The iconic Taj Mahal in Agra, one of India’s most famous landmarks, has suffered from the corrosive effects of acid rain. The acid rain has damaged the delicate white marble of the historical monument.

- Black Forest, Germany: The black forest in Germany has suffered from acid rain, which has damaged the region’s forests and affected the health of its trees.
Effects of Acid Rain
The effects of acid rain can be wide-ranging and include:
- Environmental Damage: Acid rain can harm aquatic ecosystems by making water bodies more acidic. This can lead to the decline of fish populations, damage to aquatic plants, and distribution of the food chain.
- Forest Damage: Acid rain can damage forests by leaching essential nutrients from the soil, making it harder for trees to grow. This weakens the trees and makes them more susceptible to diseases and harsh weather conditions.
- Soil Degradation: Acid rain can degrade soil quality, reducing its fertility and ability to support plant life. This can have a cascading effect on agriculture and food production.
- Corrosion of Buildings and Infrastructure: Acid rain can corrode buildings, bridges, and other structures made of metal or stone. Over time, this can lead to structural damage and costly repairs.
- Human Health: While the direct health effects of acid rain on humans are relatively minor, the pollutants that cause acid rain, such as sulfur dioxide and nitrogen oxides, can contribute to the formation of fine particulate matter (PM2.5) in the air, which is harmful to human health and can exacerbate respiratory problems.
- Economic Impact: The damage caused by acid rain can result in significant economic costs, including the repair and maintenance of infrastructure, reduced agricultural yields, and healthcare expenses related to pollution-related health issues.
Causes of Acid Rain
Acid rain is primarily caused by emissions of sulfur dioxide and nitrogen oxide into the atmosphere. Once these pollutants are released into the atmosphere, they can react with other compounds, such as water vapor and oxygen, to form sulfuric acid and nitric acid. These acids can then be transported by wind and deposited as acid rain when they come into contact with the Earth’s surface. Acid rain can have harmful effects on the environment, including damage to forests, aquatic ecosystems, and buildings.

- Burning of Fossil Fuels: The combustion of coal, oil, and natural gas in power plants, industrial facilities, and vehicles releases sulfur dioxide and nitrogen oxide into the air.
- Industrial Processes: Certain industrial activities, such as manufacturing and chemical production, can release these pollutants directly into the atmosphere.
- Transportation: Automobiles, trucks, and other vehicles emit nitrogen oxides as a byproduct of combustion.
- Agriculture: The use of fertilizers can release ammonia into the air, which can react with sulfur and nitrogen compounds in the atmosphere to form acid rain.
- Natural Sources: Volcanic eruptions and wildfires can release sulfur dioxide and nitrogen oxides into the atmosphere, although these natural sources contribute less to acid rain compared to human activities.
Preventive Measures for Acid Rain
Preventing acid rating involves reducing the emissions of sulfur dioxide and nitrogen oxides, which are the primary precursors of acid rain. Here we have mentioned some preventive measures for acid rain:
- Use cleaner energy sources: Tradition to cleaner energy sources like natural gas, wind, solar, and nuclear power, which produce fewer pollutants compared to coal and oil.
- Emission Controls: Implement strict emission control technologies in industries and power plants, such as scrubbers and catalytic converters, to reduce SO2 and NOx emissions.
- Fuel quality standards: Enforce regulations on the quality of fuels used in vehicles and industries to limit sulfur content, which reduces SO2 emissions.
- Energy Efficiency: Improve energy efficiency in industrial processes and transportation to reduce overall fuel consumption and emissions.
- Renewable energy: Promotes the use of renewable energy sources to reduce the need for fossil fuel combustion.
- Reforestation: Plant trees and preserve forests, as they can help absorb some of the pollutants responsible for acid rain.


 50 Vegetables Name for Kids in English a...
50 Vegetables Name for Kids in English a...
 Food Chain: Definition, Types, Examples,...
Food Chain: Definition, Types, Examples,...
 Human Respiratory System: Definition, Di...
Human Respiratory System: Definition, Di...













