What is Heat Capacity?
Heat capacity is a measure of how much heat energy a substance can absorb or release without a significant change in temperature. Think of it like a container’s ability to hold water – a large container can hold more water without overflowing. Similarly, a substance with high heat capacity can absorb or release more heat without experiencing a significant temperature change. Every substance has its unique heat capacity based on its composition.
For example, water has a high heat capacity, meaning it can absorb or release a lot of heat before its temperature changes noticeably. This property is crucial for regulating temperature changes noticeably. This property is crucial for regulating temperature in nature and everyday life, influencing climate patterns, and allowing our bodies to maintain a relatively stable temperature. In contrast, substances with low heat capacity, like metals, experience temperature changes more rapidly. Heat capacity is essential for understanding and managing energy transfer in various processes.
Heat Capacity Based on Thermodynamics
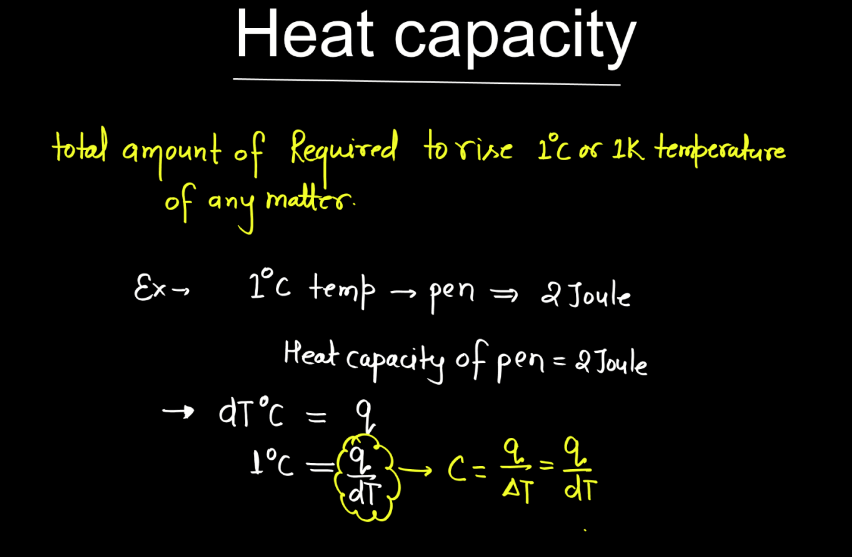
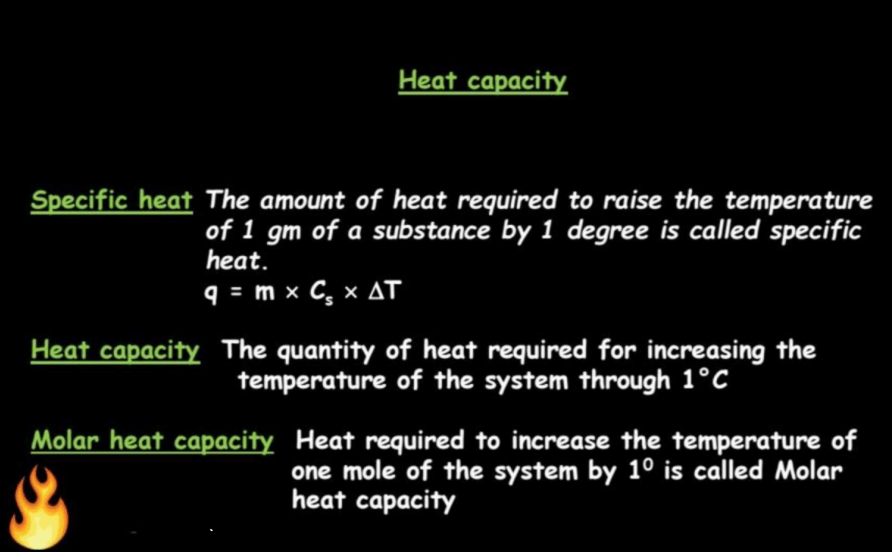
In thermodynamics, heat capacity refers to the amount of heat energy required to change the temperature of a substance by a certain amount. It is divided into two types: specific heat capacity (C or c), which is the heat energy needed to change the temperature of one unit mass of a substance by one degree Celsius, and molar heat capacity (Cm or Cn), which is the heat energy required to change the temperature of one mole of a substance by one degree Celsius. Mathematically, heat capacity is expressed as
Q = C ⋅ ΔT
Where, Q is the heat absorbed or released, C is the heat capacity, and ΔT is the change in temperature.
Understanding heat capacity is fundamental in thermodynamics as it helps analyze energy transfer in processes like heating or cooling substances. Substances with higher heat capacities can store or release more energy per temperature change, impacting the efficiency and behavior of thermodynamic systems.
Components of Heat Capacity
There are two main components of specific heat capacity. These components help describe how a substance responds to changes in temperature and the amount of heat energy it can absorb or release.
| Components of Heat Capacity | |
| Components | Description |
| Specific Heat Capacity (C) | This is the amount of heat required to raise the temperature of a unit mass (usually one gram or one kilogram) of a substance by one degree Celsius (or one Kelvin). |
| Molar Heat Capacity | This is the heat capacity per mole of a substance. It is calculated by multiplying the specific heat capacity by the molar mass of the substance. |
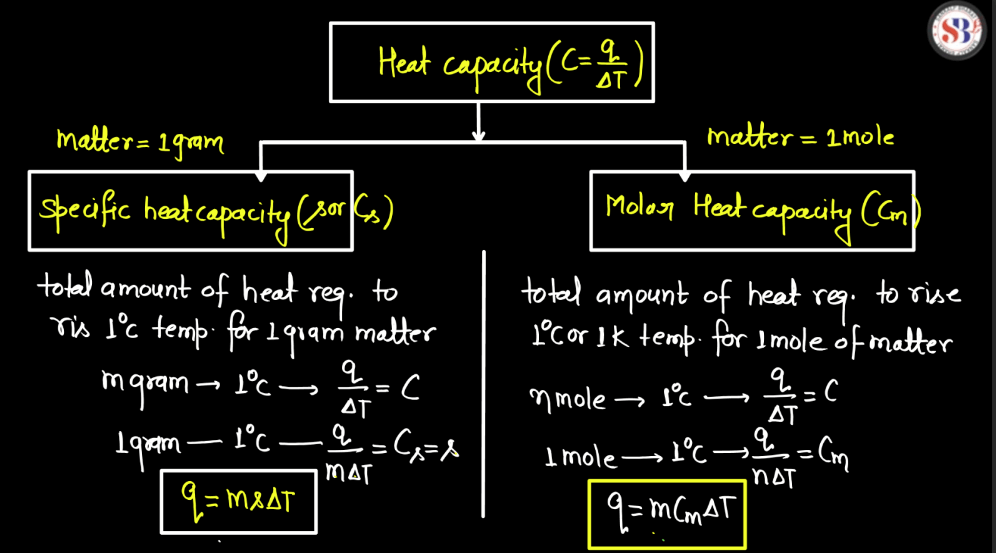
Specific Heat Capacity
Specific heat capacity is a measure of how much heat energy is needed to raise the temperature of a certain amount of substance by a certain amount. Imagine heating a cup of water on a stove – the specific heat capacity tells us how much energy is required to make that water hotter. Every substance has its own unique specific heat capacity because different materials respond differently to heat. In simple terms, think of a specific heat as the “recipe” for heating or cooling a substance. The formula for specific heat is expressed as:
Q = m ⋅ C ⋅ ΔT
Where Q is the heat energy absorbed or released, m is the mass of the substance, C is the specific heat, and ΔT is the temperature change.
It’s like saying, “To make the water hotter, you need this much heat energy”. Water has a relatively high specific heat capacity, meaning it requires a lot of heat to change its temperature. This is why water takes longer to heat up or cool down compared to some other substances. Mathematically, specific heat capacity is expressed as the amount of heat energy (measured in joules) required to raise the temperature of one kilogram of the substance by one degree Celsius. So, it’s like understanding the energy needed to make a specific amount of material get warmer or cooler.
Molar Heat Capacity
Molar heat capacity is a measure of how much heat energy is needed to raise the temperature of one mole of a substance by one degree Celsius (or one Kelvin). It provides insight into a material’s ability to store thermal energy. Think of it like a container’s capability to hold water-molar heat capacity tells us how much heat a substance can “hold” per mole. Specifically, there are two types of molar heat capacity: at constant volume (Cv) and at constant pressure (Cp).
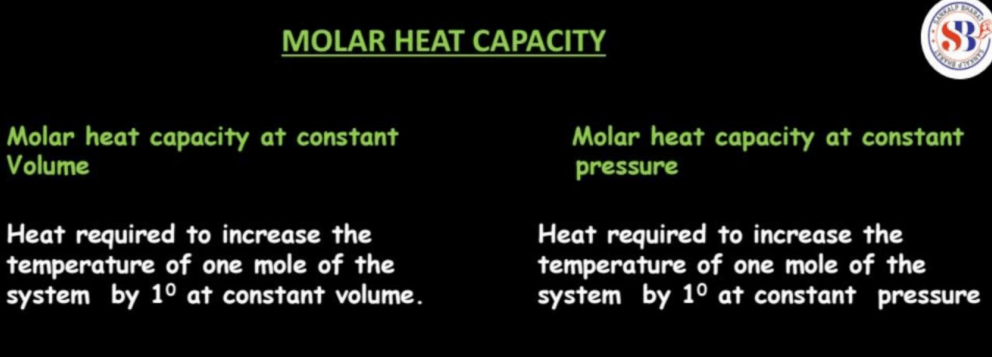
Cv is the amount of heat needed to raise the temperature of one mole while keeping the volume constant, while Cp considers changes in pressure. These values are crucial in understanding and predicting how substances respond to changes in temperature. In simple terms, molar heat capacity helps us grasp how substances absorb and release heat during temperature changes, essential in fields like chemistry and physics for understanding the behavior of materials in various conditions.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













