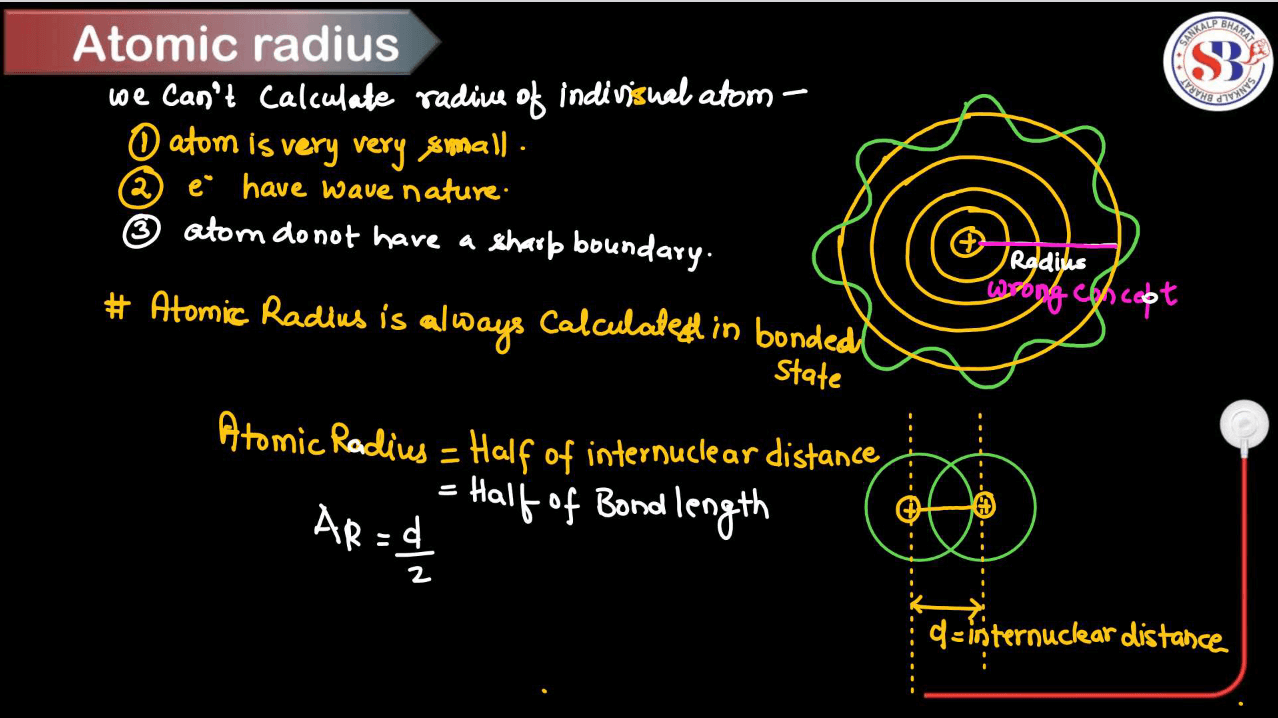
An atom is the smallest unit of matter that retains the properties of an element. It consists of a nucleus, which contains protons and usually neutrons, surrounded by a cloud of electrons. Protons carry a positive charge, electrons carry a negative charge, and neutrons have no change. The number of protons determines the element’s identity, while the number of electrons determines its chemical properties. The total distance from the nucleus of an atom to the outermost orbital of its electron is known as its atomic radius. Atoms combine molecules, which are the building blocks of all substances in the universe. For more information regarding Atomic Radius go through the complete article below.
What is Atomic Radius?
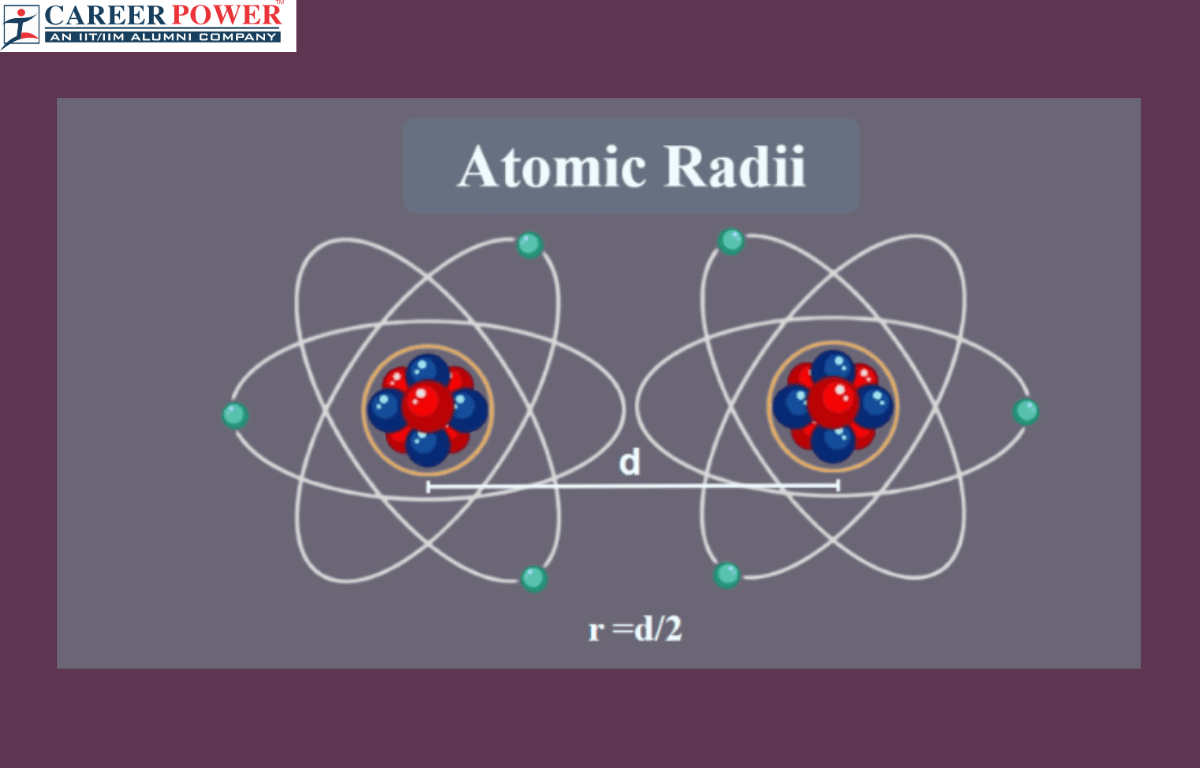
An atomic radius, also known as atomic radii, is the distance from the nucleus of an atom to the outermost electron shell. Picture an atom as a tiny solar system, with electrons orbiting the nucleus like planets around the sun. The atomic radius measures how far these outer electrons are from the nucleus, essentially the size of the atom. Think of it like the size of a planet’s orbit around the sun – closer orbits mean a smaller atomic radius, while farther orbitals mean a larger atomic radius. Elements with more electrons have larger atomic radii because their outer electrons are farther from the nucleus. So, atomic radius tells us about an atom’s size and how its electrons are distributed around its nucleus.
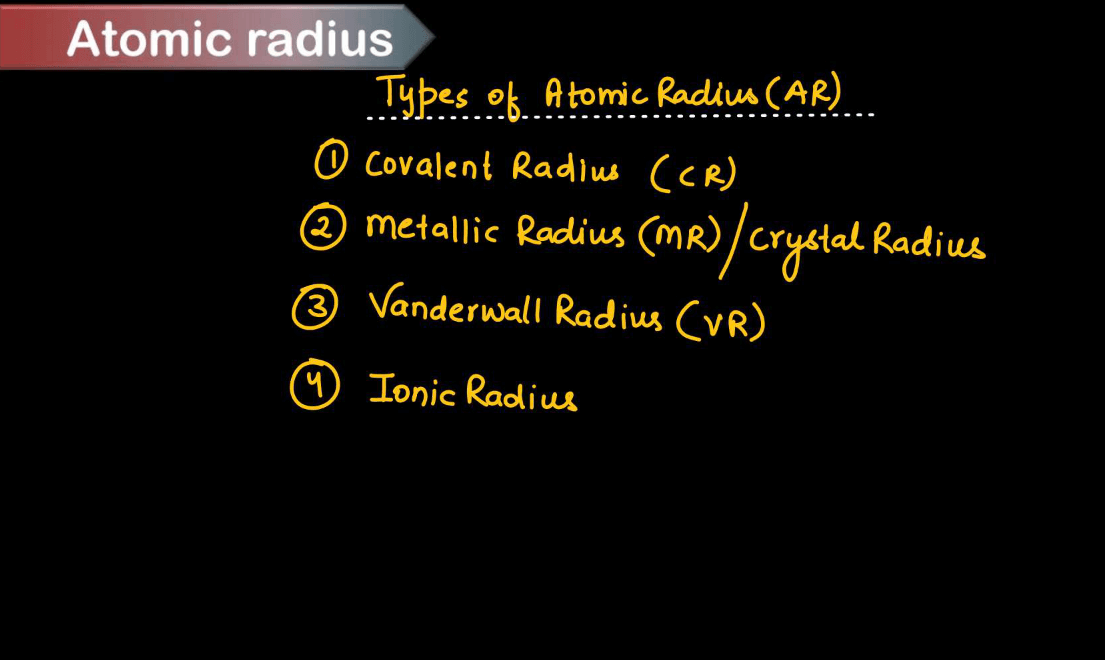
Types of Atomic Radius
Atomic radius refers to the distance from the nucleus of an atom to the outermost electron orbital. The types of atomic radius can be understood in the context of different types of chemical bonds. Each of these types of atomic radius provides insight into the size of atoms and ions in different bonding environments, helping to understand and predict the behavior of chemical compounds.
| Different Types of Atomic Radius | |
| Types | Description |
| Covalent Radius | This is the radius of an atom when it forms a covalent bond, atoms share electron pairs, so the covalent radius is half of the distance between the nuclei of two bonded atoms. |
| Ionic Radius | Ionic radius refers to the size of an ion in an ionic compound. When an atom loses or gains electrons to form an ion, its size changes. Cations (positively charged ions) are smaller than their parent atoms because they lose electrons, while anions (negatively charged ions) are larger because they gain electrons. Thus, the ionic radius depends on the nature and charge of ions |
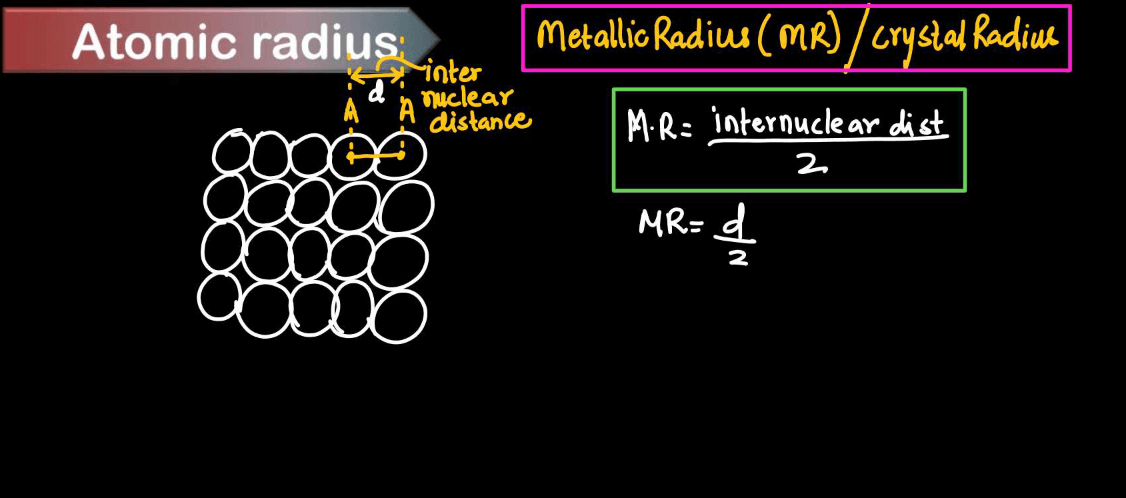
| Metallic Radius | Metallic radius refers to the size of atoms in metallic elements when they are packed together in a metallic lattice. In metals, atoms are arranged in a closely packed structure and the metallic radius is half the distance between the nuclei of adjacent atoms. |
| Van der Waal Radius | This is the radius of an atom or molecule in a non-bonded state, where it interacts with other atoms or molecules through van der Waals forces. Van der Waals radius is typically larger than covalent or ionic radii because it accounts for the size of the entire atom or molecule, including any electron clouds that extend beyond the outermost electron orbitals |
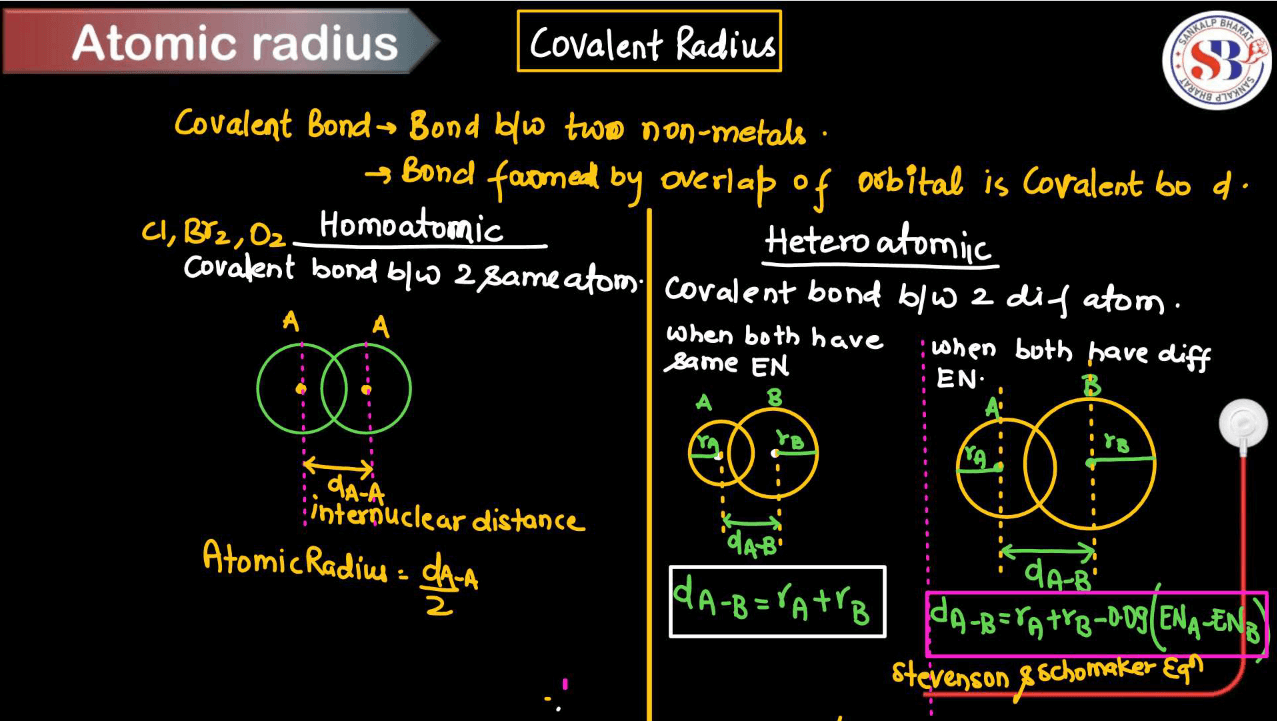
Covalent Radius
A covalent radius is like an imaginary boundary that represents how close two atoms can get to each other when they’re bonded together by sharing electrons. Picture two atoms in a molecule, holding hands by sharing some of their electrons. The covalent radius measures the distance from the center of one atom to where the bond ends with the other. It’s like the comfortable personal space they maintain while still being connected. This radius varies depending on the types of atoms involved and the nature of their bonds. Essentially, it’s a way to quantify the size of atoms when they’re bonded together in a molecule.
Ionic Radius
Ionic radius is like the size of an atom when it forms an ion by gaining or losing electrons. Imagine an atom as a little ball. When it loses electrons, it becomes a positively charged ion, and its radius shrinks because it has fewer electron layers. Conversely, when it gains electrons, it becomes a negatively charged ion, and its radius expands because it now has more electron layers. So, the ionic radius is the distance from the center of the ion to its outermost electron or electron shell. It helps us understand how the size of atoms changes when they form ions.
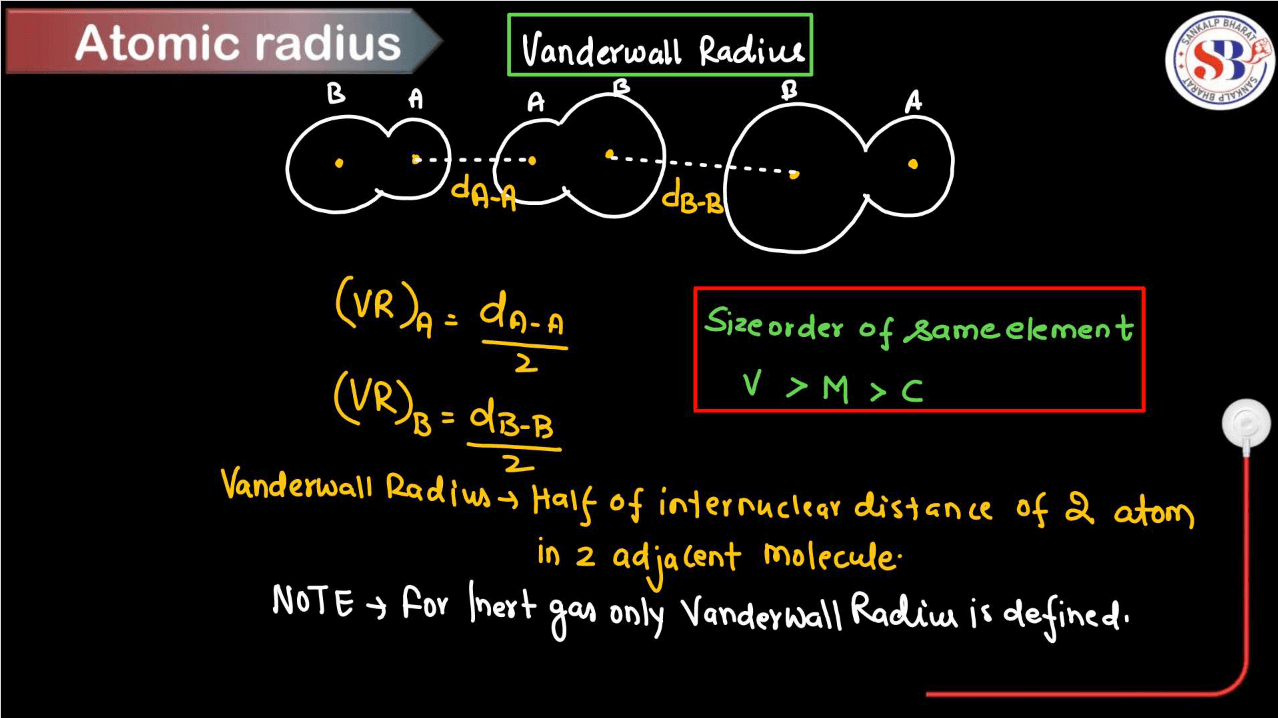
Van der Waal Radius
The van der Waals radius is like the invisible cushion around an atom, representing its size when it’s not bonded to another atom. Think of it as the distance from the nucleus where the atom’s influence ends. It’s a measure of how close atoms can get to each other without bonding. Picture two balls; their van der Walls radii are the closest they can roll past each other without touching. This helps understand molecular structures, like predicting how molecules might stack or interact. In essence, it’s a handy way to imagine the personal space atoms need to maintain around themselves.
Metallic Radius
Metallic radius is like the size of an atom in a metal, where atoms are arranged in a closely packed lattice structure. Think of a stack of balls in a container, each representing an atom. The metallic radius is the distance from the center of one atom to the center of its neighboring atoms in this structure. It’s like measuring how closely packed the atoms are within the metal. Since metals tend to share electrons freely and form a sea of electrons, the metallic radius reflects the average distance between atoms in a metal’s solid state.
Bohr Radius
The Bohr radius is like an invisible boundary around an atom’s nucleus, determining its size. Imagine the nucleus as a tiny, dense core, and the Bohr radius as the average distance where electrons typically orbit around it, like planets around the sun. It’s a crucial concept in understanding atomic structure. The size of this boundary varies depending on the type of atom. For hydrogen, it’s about 0.5 angstroms, roughly the width of a single atom. Essentially, it’s a fundamental measure of an atom’s size, helping us grasp the scale and organization of the microscopic world.
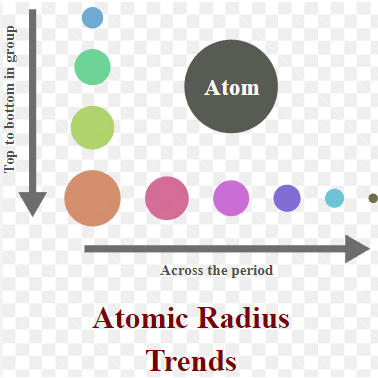
Periodic Trends of Atomic Radii
Atomic radii, a measure of an atom’s size, follow predictable trends across the periodic table. Generally, as you move from left to right across a period (horizontal row), atomic radii decrease. This happens because as more protons are added to the nucleus, they pull the electrons closer, shrinking the atomic size. Conversely, as you go down a group (vertical column), atomic radii increase. This is because each subsequent row adds another electron shell, expanding the atom’s size. So, atoms on the bottom left of the periodic table tend to be the largest, while those on the top right are the smallest.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













