CBSE Class 12 Chemistry Answer Key 2024: The Central Board of Secondary Education has conducted the CBSE Class 12 Chemistry Exam 2024 on February 27, 2024 at various exam centers. The Class 12 Chemistry exam was from 10:30 am to 01:30 pm. The students were provided an extra 15 minutes to read the question paper. As the Class 12 Chemistry exam has been conducted all the students who appeared for the CBSE Chemistry Board Examination 2024 can be able to check the complete CBSE Class 12 Chemistry Answer Key 2024 PDF with Question Paper All Set 1, 2, 3, and 4 via direct link given in the article below.
CBSE Class 12 Chemistry Answer Key 2024
The CBSE Class 12th Chemistry Board Examination 2024 was scheduled to be conducted on February 27, 2024. Students were allotted a time duration of 3 hours to complete the exam whereas an extra 15 minutes will be given for reading the question paper. Chemistry is always a challenging subject for students due to its vast scope and both descriptive and mathematical nature. After the successful conduction of the CBSE Class 12 Chemistry Exam 2024 students can check the complete Class 12 Chemistry answer key 2024 here. However students must understand that the answer key may not always be accurate, so cross-checking with the official sources is very important.
Class 12 Chemistry Answer Key 2024
The CBSE Class 12th Chemistry examination 2024 comprises a total of 100 marks out of which 70 marks will be for the Class 12th Chemistry theory exam while the rest 30 marks will be for the Chemistry Practical exam and viva.
| CBSE Class 12 Chemistry Answer Key 2024 | |
| Exam Conducting Body | Central Board of Secondary Education |
| Name of the Examination | CBSE Class 12th Board Examination 2024 |
| Category | Answer Key |
| Status | Released |
| CBSE Class 12 Chemistry Exam Date 2024 | February 27, 2024 |
| Class 12 Chemistry Answer Key 2024 Release Date | February 27, 2024 |
| Chemistry 12th Total Marks | 70 marks |
| Negative Marking | No negative Marking |
| Mode of Answer Key Availability | Online Mode |
| Official Website | https://www.cbse.nic.in/ |
Class 12 Chemistry CBSE Answer Key 2024 All Sets
We here provided all the SET 1, 2, 3, 4 Class 12 Chemistry Answer Key 2024 for students to help them analyse the exam paper.
CBSE Class 12 Chemistry Answer Key 2024 SET 1 56/2/1
SECTION A
1. When MnO2 is fused with KOH in air, it gives
(A) KMnO4
(B) K2MnO4
(C) MngO7
(D) Mn2O
Answer: (B) K2MnO4
2. Ligand EDTA is an example of a:
(A) Monodentate ligand
(B) Didentate ligand
(C) Tridentate ligand
(D) Polydentate ligand
Answer:(D) Polydentate ligand
3. Which of the following ligand forms chelate complex?
(A) C2O2-4
(B) CI
(C) NO
(D) NH3
Answer: (A) C2O2-4

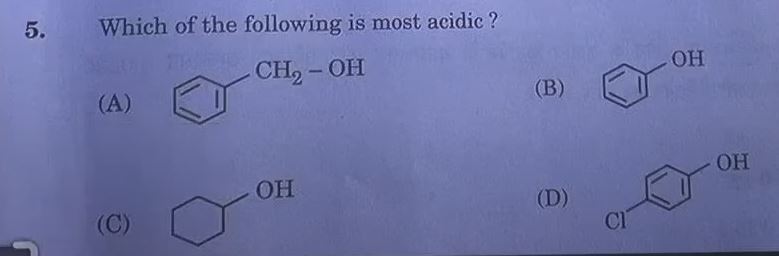
Answer: (C)
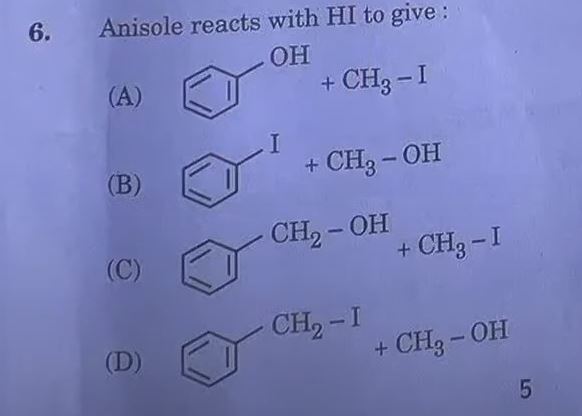
Answer: (B)
Answer: (A)
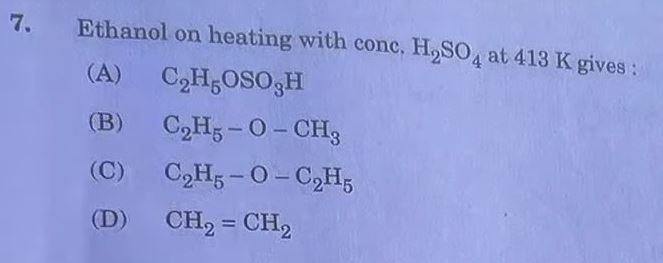
Answer: (D)
8. An Azetrophic Solution of two liquids has boiling point lower than either of them when it
(A) is saturated
(B) shows positive deviation from Raoult’s law
(C) shows negative deviation from Raoult’s law
(D) shows no deviation from Raoult’s law
Answer: (B) shows positive deviation from Raoult’s law
9. The relative lowering of vapour pressure of an aqueous solution containing non-volatile solute is 0.0225. The mole fraction of the non-volatile solute is:
(A) 0.80
(B) 0.725
(C) 0:15
(D) 0.0225
Answer: (D) 0.0225
10. During electrolysis of aqueous solution of NaCl :
(A) H2 (g) is liberated at cathode
(B) Na is formed at cathode
(C) O2 (g) is liberated at anode
(D) Cl2 (g) is liberated at cathode
Answer: (A) H2 (g) is liberated at cathode
11. The addition of catalyst during a chemical renètion alters which of the following quantities of the reaction?
(A) Enthalpy
(B) Activation energy
(C) Entropy
(D) Internal energy
Answer: (B) Activation Energy
12. For the elementary reaction PQ, the rate of disappearance of ‘P’ increases by a factor of 8 upon doubling the concentration of ‘P’. The order of the reaction with respect to ‘P’ is:
(A) 3
(B) 4
(C) 2
(D) 1
Answer: (A) 3
13.
Assertion (A): Aliphatic primary amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Alkyl halides undergo nucleophilie substitution with anion formed by phthalimide.
Answer: (A) Both true
14.
Assertion (A): Uracil base is present in DNA.
Reason (R): DNA undergoes self-replication.
Answer: (D) Assertion is False, But Reason is True
15.
Assertion (A): Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Reason (R): Diazonium salts of aliphatic amines show resonance.
Answer: (C)
16.
Assertion (A): p-nitroaniline is a weaker base than p-toluidine.
Reason (R): The electron withdrawing effect of NO2 group in p-nitroaniline makes it a weaker base.
Answer: (A) Both true
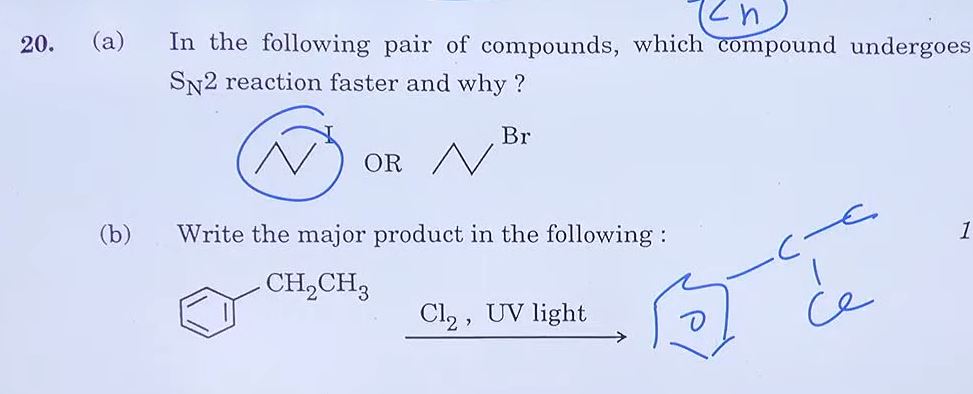
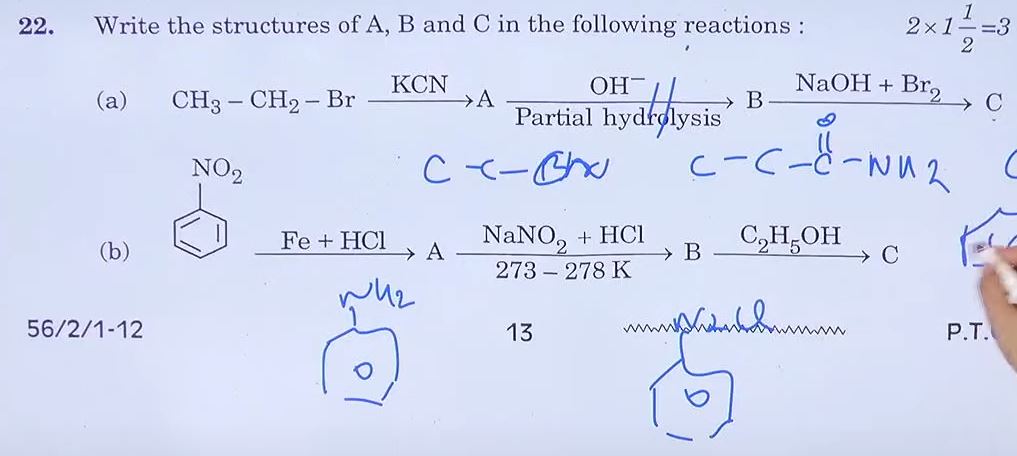
21. Define the following terms:
(a) Denaturation of protein
Answer: Denaturation of Protein refers to the alteration of the native structure of a protein molecule without breaking its peptide bonds. This process typically involves the disruption of the secondary, tertiary, or quaternary structures of the protein, resulting in the loss of its biological activity. Denaturation can be caused by various factors such as heat, extreme pH levels, organic solvents, or mechanical agitation. During denaturation, the protein unfolds and loses its characteristic three-dimensional shape, which is essential for its function.
(b) Invert Sugar
Answer: Invert sugar is a mixture of two simple sugars: glucose and fructose. It is formed by the hydrolysis (breakdown) of sucrose, which is a disaccharide composed of one molecule of glucose and one molecule of fructose linked together.
CBSE Class 12 Chemistry Answer Key 2024 SET 2 56/4/2
SECTION A
1. The molar ionic conductivities of Mg2+ and SO2 are 106.0 S cm² mol-¹ and 160.0 S cm² mol-¹ respectively. The value of limiting molar conductivity of MgSO4 will be :
(A) 266 S cm² mol-1
(C) 288 S cm² mol-1
(B) 622 S cm² mol-1
(D) 822 S cm² mol-1
Answer: (A) 266 S cm² mol-1
2. From the elements of 3d series given below, which element shows the maximum number of oxidation states?
(A) Scandium
(C) Chromium
(B) Manganese
(D) Titanium
Answer: (B) Manganese

Answer: (A)
4. Which of the following acids represent Vitamin C?
(A) Saccharic Acid
(B) Gulconic Acid
(C) Ascorbic Acid
(D) Benzoic Acid
Answer: (C) Ascorbic Acid

Answer: (A)

Answer: (A) A – Methanol, B – Potassium Formate
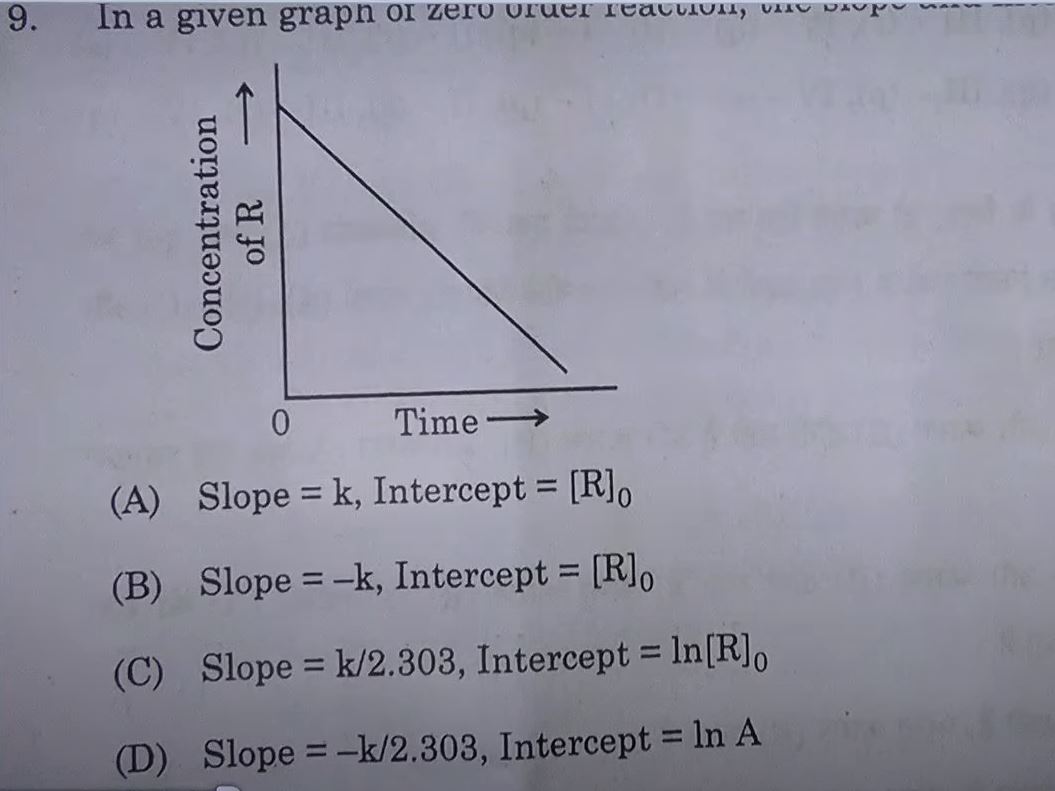
7. In effective collisions the colliding molecules must have :
(A) Proper orientation only
(B) A certain minimum amount of activation energy.
(C) Threshold energy only.
(D) Threshold energy and proper orientation both.
Answer: (D) Threshold energy and proper orientation both.
8. Identify the secondary amine from the given options:
(A) (CH3)2CHΝΗ2
(B) CH3NHCH(CH3)2
(D) CH3(CH2)2NH2
(C) (CH3)3CNH2
Answer: (B) CH3NHCH(CH3)2
Answer: (A)
10. The general electronic configuration of d-block elements is:
(A) (n-1) d1-10ns1-2
(B) (n-1) d10ns1-2
(C) (n-1) d10ns2-3
(D) (n-1) d0ns1-2
Answer: (A) (n-1) d1-10ns1-2
11. Nucleophilic addition of Grignard reagent of Ketones followed by hydrolysis with dilute acids forms.
(A) Alkene
(B) Primary Alcohol
(C) Tertiary Alcohol
(D) Secondary Alcohol
Answer: (C) Tertiary Alcohol

Answer: (A)
13. Assertion (A): Bromination of Phenol can be carried out even in the absence of Lewis acid.
Reason (R): – OH group of Phenol has the high activation effect
Answer: (A) both are true
14. Assertion (A): Fructose is a reducing sugar.
Reason (R): Fructose does not reduce Fehling solution and Tollen’s reagent.
Answer: (A) Both are True
15. Assertion (A): For a Daniell cell, Zn/Zn2+(1M) || Cu2+ (1M)/Cu Ecell = 1.1 V, if the external opposing potential is more than the electrons flow from Cu to Zn.
Reason (R): Cell acts like a galvanic cell
Answer:
16. Assertion (A): Benzoic Acid does not undergo Friedel Crafts reaction
Reason (R): Carboxyl group is deactivating and the catalyst aluminum chloride gets bonded to the carboxyl group
Answer:
CBSE Class 12 Chemistry Answer Key 2024 – MCQs
Class 12 Chemistry Board Exam Section A comprises 16 multiple-choice questions, each worth one point. In the table below, we have mentioned the correct answer for each MCQs asked in CBSE Class 12 Chemistry Exam 2024.
| CBSE Class 12 Chemistry Answer Key 2024 All SET (MCQ Section A) | |||
| Question Number | SET 1 (56/2/1-12) | SET 2 (56/4/2) | SET 3 |
| 1 | B | A | – |
| 2 | D | B | – |
| 3 | A | A | – |
| 4 | C | C | – |
| 5 | B | A | – |
| 6 | A | A | – |
| 7 | D | D | – |
| 8 | B | B | – |
| 9 | D | A | – |
| 10 | A | A | – |
| 11 | B | C | – |
| 12 | A | A | – |
| 13 | A | A | – |
| 14 | D | A | – |
| 15 | C | – | |
| 16 | A | – | |
CBSE Class 12 Chemistry Question Paper 2024 PDF Download
The Central Board of Secondary Education has conducted the Class 12th Chemistry Board Exam 2024 on February 27, 2024. The CBSE Board Question Papers are valuable resources as after the successful conduction of the exam, these question papers help the students to evaluate their performance in the exams. These question papers will help the students preparing to appear in the class 12th exams next year to provide an overview of the question paper, and the types of questions asked in the examination. The direct link to download the CBSE Class 12th Chemistry Question Paper 2024 PDF has been shared here.
| CBSE Class 12 Chemistry Question Paper 2024 PDF | |
| Class 12 Chemistry Exam Paper 2024 SET | Question Paper Link |
| SET 1 | Class 12 Chemistry SET 1 Question Paper 2024 |
| SET 2 | Class 12 Chemistry SET 2 Question Paper 2024 |
| SET 3 | Class 12 Chemistry SET 3 Question Paper 2024 |
CBSE Class 12 Chemistry Answer Key 2024 PDF Download
All the students who are appearing in the Class 12 Chemistry exam will be able to estimate their board marks via CBSE Class 12 Chemistry Answer Key 2024. The evaluation via the answer key can help students predict their marks. The official exam conducting authorities will not release any answer key for the CBSE Class 12 Chemistry Question Paper 2024, but our subject expert’s faculty will release the specially designed answer key for the questions that will be asked in the CBSE Class 12 Chemistry Exam. All the students who appeared for the CBSE Class 12th Chemistry Board Exam 2024 can able to check the complete CBSE Class 12th Chemistry Answer Key 2024 PDF from the direct link that has been shared in our article.
CBSE Class 12 Chemistry Exam Paper Analysis 2024
Most of the Experts and educators have mentioned that the CBSE Class 12th chemistry paper was balanced and had a moderate difficulty level. Most of the questions were direct and based on NCERT. Students who appeared for the chemistry exam today i.e., February 27, 2024 mentioned that all questions were based on the syllabus and followed the same format as the latest CBSE class 12th Chemistry sample paper. The majority of the questions in the paper ranged from easy to moderate, except for a few that could have posed a challenge for students. Overall, the CBSE Class 12 Chemistry paper was quite average for almost all the students
Shreyas Singh from DAV Public School said that the Chemistry paper was somewhat tricky. He noted, “All questions were directly sourced from the NCERT textbooks.” Additionally, he mentioned, “Mock tests played a crucial role in aiding my approach to tackling the paper.”
Sanvi and Harshit said, “that in CBSE Class 12th Chemistry question paper section B was reasoning based and some incomplete equations were difficult.” Aryan found the paper lengthy, and some tricky equation-based questions consumed a lot of time
- The overall difficulty level of the CBSE Class 12th Chemistry Question Paper 2024 ranged from easy to moderate.
- According to the Class 12th students, the Multiple Choice Questions part of the chemistry paper was slightly tricky but manageable.
- All the questions in Section B were easy.
- Questions in section C were direct.
- Case-based questions in section D were moderate.
- Long answer type questions in the paper were direct.
CBSE Class 12 Chemistry Exam Analysis 2024
All the students who appeared in the Class 12 Chemistry Board Exam 2024 can check the complete CBSE Class 12 Chemistry Exam Analysis 2024 for all SET 1, 2, 3, 4. Watch the complete video:



 CBSE Class 10th Maths Answer Key 2026, C...
CBSE Class 10th Maths Answer Key 2026, C...
 CUET Legal Study Previous Year Question ...
CUET Legal Study Previous Year Question ...
 NIMCET Final Answer Key 2025 Out, Downlo...
NIMCET Final Answer Key 2025 Out, Downlo...













