Theory of Photon
The photon theory posits that light is composed of discrete packets of energy called photons. Proposed by Albert Einstein in 1905, this theory revolutionized our understanding of light. Protons are tiny, indivisible particles with no mass that carry electromagnetic energy. They behave both as particles with no mass that carry electromagnetic energy. They behave both as particles and waves, exhibiting characteristics like interference and diffraction. When light interacts with matter, such as during the photoelectric effect, photons transfer their energy to electrons, causing them to be emitted. The quantization of light into photons explains various phenomena, including the particulate nature of light and the relationship between light intensity and energy. In essence, the photon theory provides a foundational framework for comprehending the fundamental nature of light.




Different Experiments
Now let us explore a few experiments such as the Hertz Experiment, Hallwachs Experiment, and Lenard Experiment. Each of these experiments contributed to the advancement of physics during the late 19th and early 20th centuries, providing empirical support for theoretical concepts and laying the groundwork for the quantum revolution.
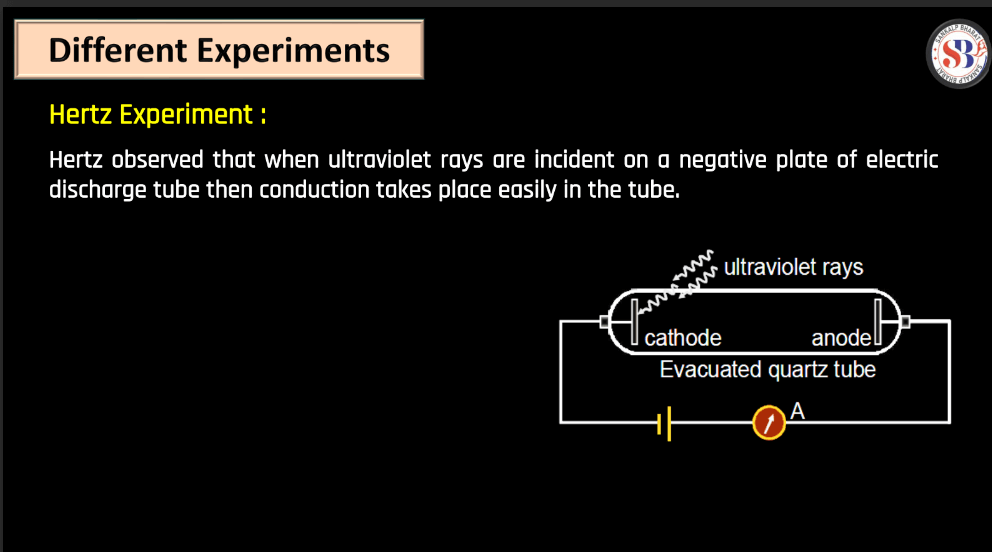
Hertz Experiment (1887)
- Description: Heinrich Hertz conducted experiments to verify the existence of electromagnetic waves predicted by James Clerk Maxwell. He generated and detected radio waves using an oscillator and a spark gap.
- Significance: Hertz’s experiments provide experimental confirmation of Maxwell’s theory and laid the foundation for the development of wireless communication technology.
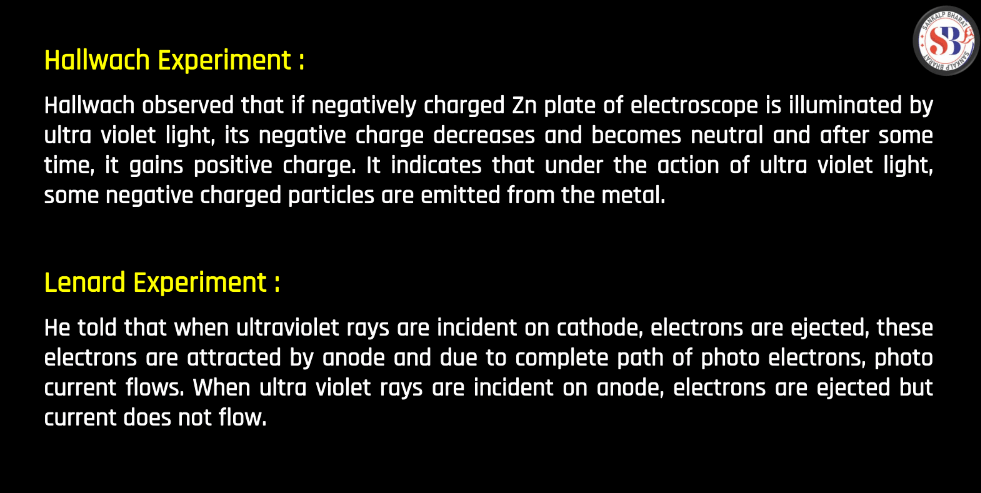
Hallwachs Experiment (1888)
- Description: Wilhelm Hallwachs conducted experiments involving the photoelectric effect. He observed that a charged zinc plate, exposed to ultraviolet light, lost its charge more ultraviolet light, lost its charge more quickly, indicating the release of charged particles (photoelectron).
- Significance: Hallwatch’s work contributed to the understanding of the photoelectric effect, a phenomenon crucial for later developments in quantum theory.
Lenard Experiment (1902-1905)
- Description: Physicist Philipp Lenard investigated the photoelectric effect in detail. He studied how the kinetic energy of emitted photoelectrons depended on the frequency and intensity of incident light.
- Significance: Lenard’s experiments provided crucial data for Albert Einstein’s 1905 explanation of the photoelectric effect, which played a significant role in the development of quantum theory.
Photoelectric Effect
The photoelectric effect is a physics phenomenon where light, typically in the form of photons, causes the emission of electrons from a material’s surface. When light strikes the material’s surface. When light strikes the material, photons transfer their energy to electrons, enabling them to overcome the material’s binding forces and be ejected. Notably explained by Albert Einstein, this effect contributed to the understanding of the particle-like nature of light and laid the foundation for quantum theory. The photoelectric effect has practical applications in technologies like photovoltaic cells, where it is harnessed for converting light energy into electrical energy.

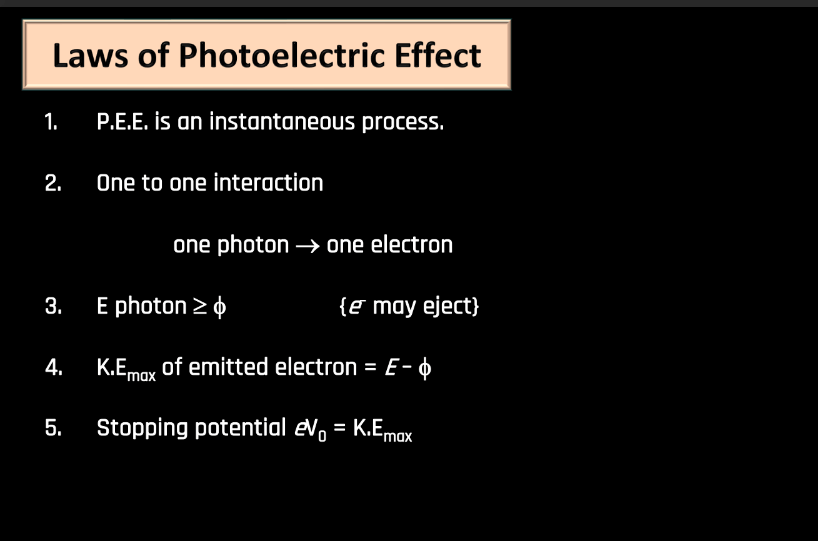
Laws of Photoelectric Effect
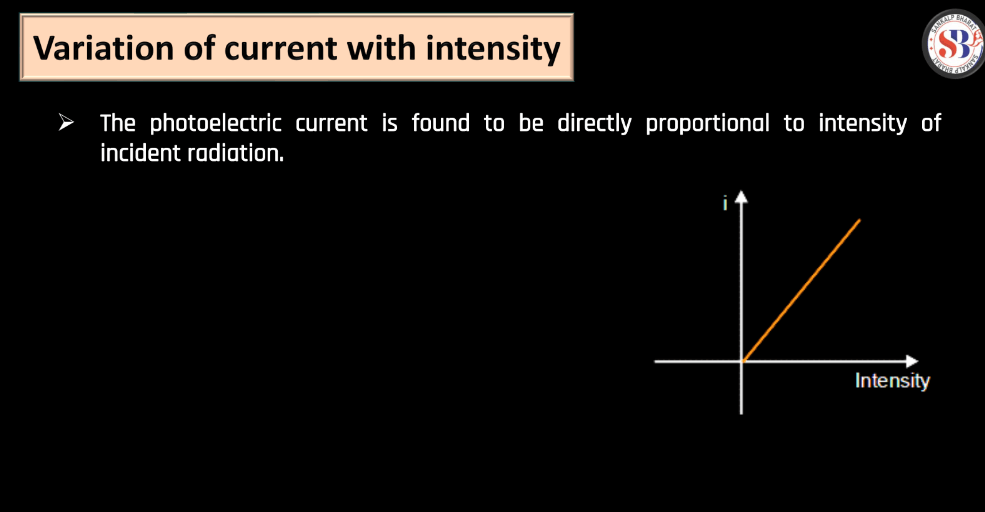
The photoelectric effect involves the emission of electrons from a material when exposed to light. Below we have discussed a few of them have been below. All these concepts were crucial in Einstein’s explanation of the photoelectric effect and contributed significantly to the development of quantum theory.
- Instantaneous Process (P.E.E): Photoemission occurs immediately when light of sufficient frequency strikes a material, with no time delay.
- One-to-One Interaction: Each photon interacts with a single electron. This is a fundamental principle in the photoelectric effect.
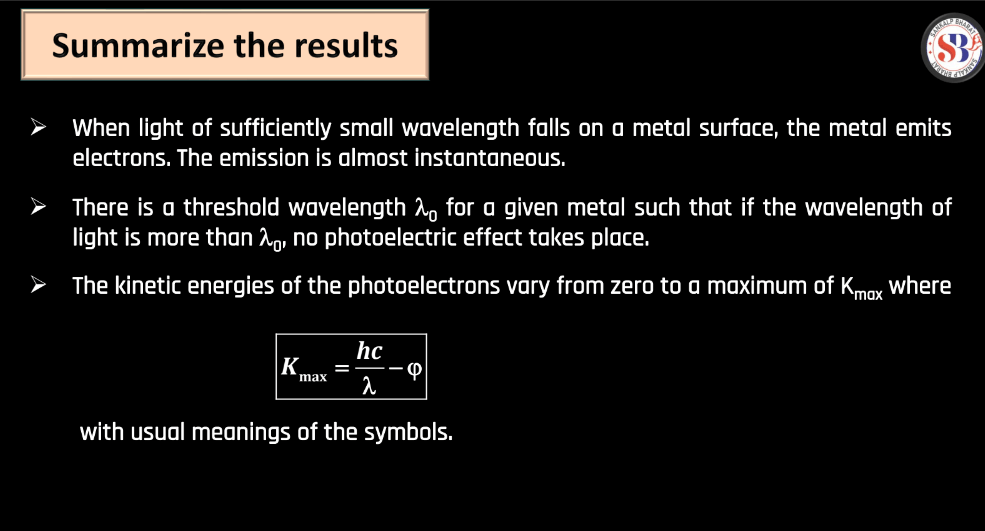
- Kinetic Energy (K.Emax) of Emitted Electrons: The maximum kinetic energy of emitted electrons is determined by the energy of the incident photons (E) minus the work function of the material.
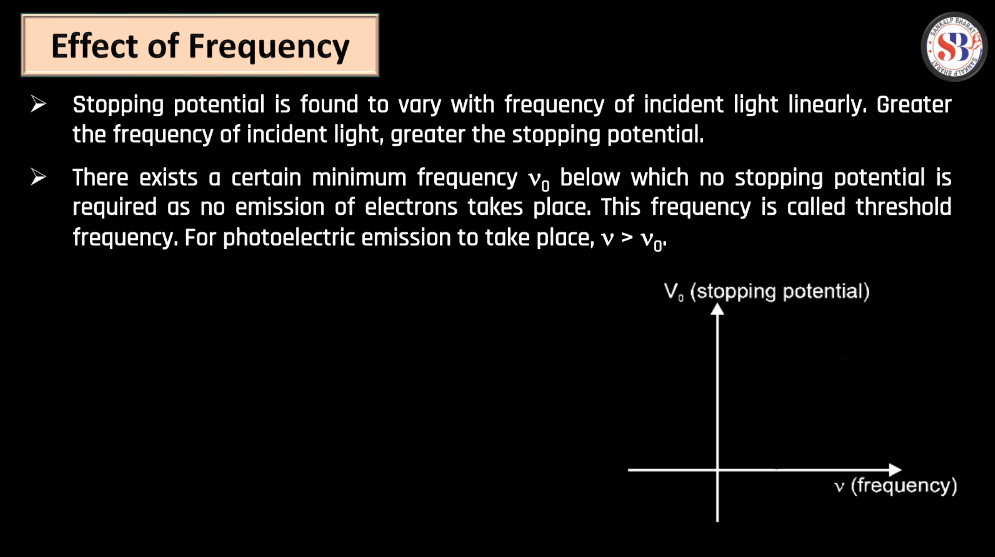
- Stopping Potential eV0 = K.Emax: The stopping potential (V0) is the minimum potential applied to stop the flow of electrons emitted in the photoelectric effect. In a scenario where V0 is equal to the minimum kinetic energy of the emitted electrons (K.Emax), the kinetic energy of the electrons is completely counteracted, resulting in no current flow.




Work Function
The work function represents the minimum energy needed to liberate an electron from a material’s surface. Measured in electron volts (eV), it quantifies the ease of electron emission, crucial in phenomena like the photoelectric effect or vacuum tube electron release. When light or other forms of energy strike a material, electrons can be elected if their energy exceeds the work function. This concept is fundamental in understanding electron behavior at surfaces and plays a pivotal role in various applications, including semiconductor devices and photoelectric cells.

Threshold Wavelength
Threshold wavelength is the wavelength is the specific color of light, or wavelength, that triggers a particular reaction or phenomenon. In simpler terms, it’s like the minimum amount of energy needed to kickstart a certain process. For example, in the minimum wavelength required to release electrons from a material. Beyond this threshold, the material responds; below it, nothing happens. Think of it as a key that fits into a lock – only when you have the right key (wavelength) does the lock (reaction) open. Understanding threshold wavelength helps in various specific fields, from electronics to chemistry, where specific wavelengths of light play a crucial role in initiating specific reactions or behaviors.

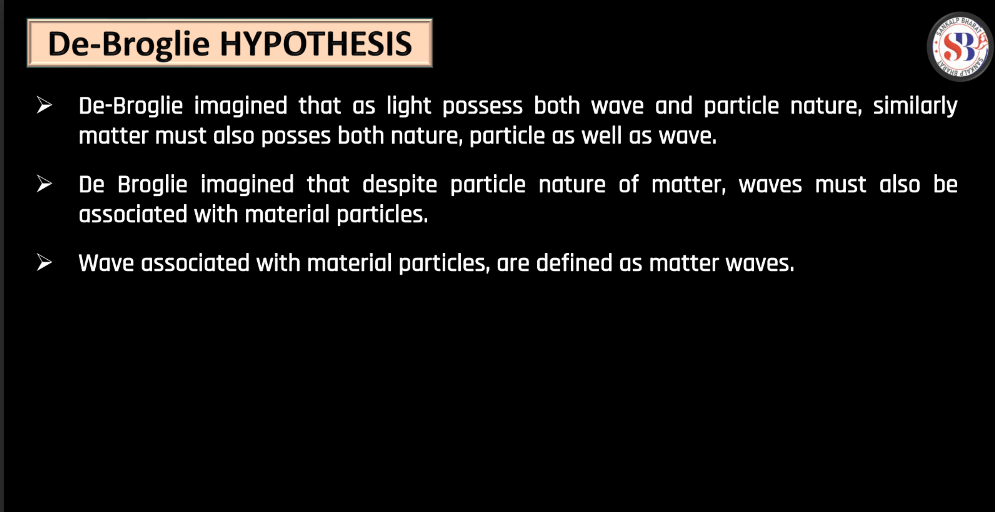
De Broglie Hypothesis
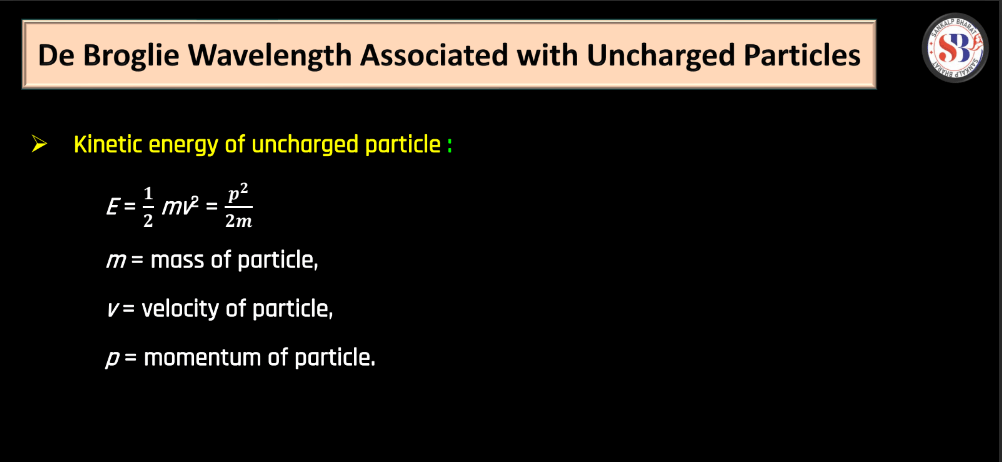
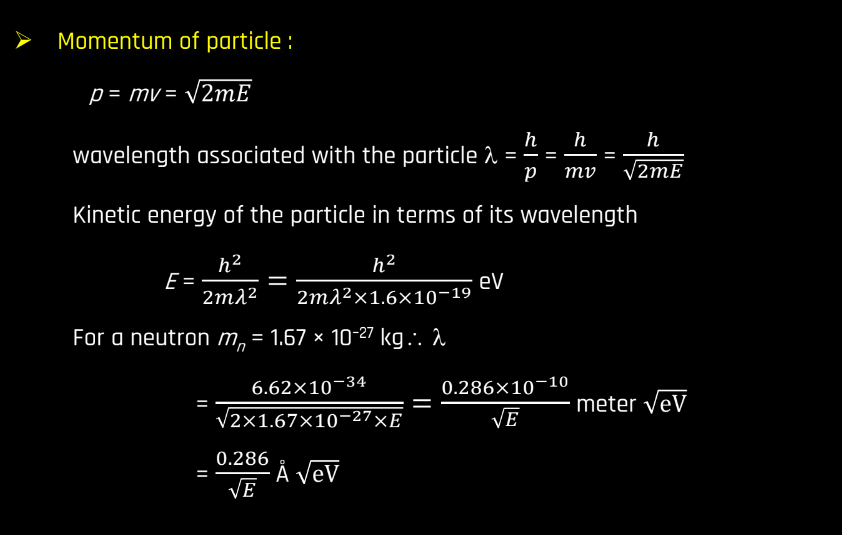
The De Broglie hypothesis, proposed by French physicist Louis de Broglie in 1924, suggests that particles, like electrons, exhibit both particle and wave-like properties. De Broglie proposed that every moving particle has a corresponding wave associated with it. The wavelength of this wave is inversely proportional to the particle’s momentum. This relationship is expressed by the De Broglie equation: λ = h/p
Where, λ is the wavelength, h is Plank’s constant, and p is the momentum of the particle. Essentially, the hypothesis implies that even though particles like electrons are traditionally thought of as tiny, discrete entities, they also display wave characteristics. This also displays wave characteristics. This duality was a groundbreaking concept, challenging classical notions and laying the foundation for the development of quantum mechanics. The de Broglie hypothesis plays a crucial role in understanding the behavior of particles on a microscopic scale.

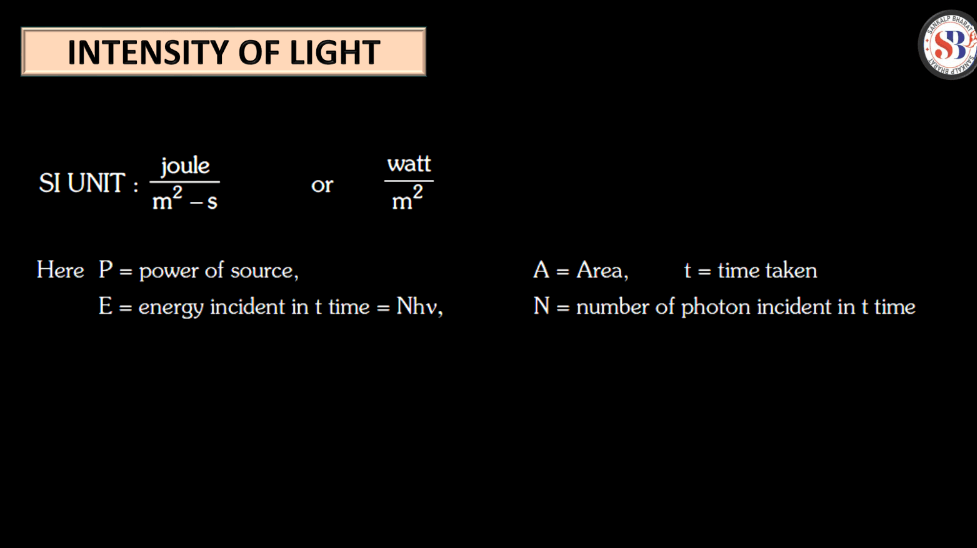
Intensity of Light
Intensity of light refers to the amount of energy carried by light waves per unit of space. Imagine light as a stream of tiny particles called photons. The intensity is like the number of photons passing through a specific area. Bright light has high intensity, while dim light has low intensity. Intensity is measured in units like watts per square meter. Think of it as the brightness knob on a lamp – turning intensity, while a cloudy has lower intensity.
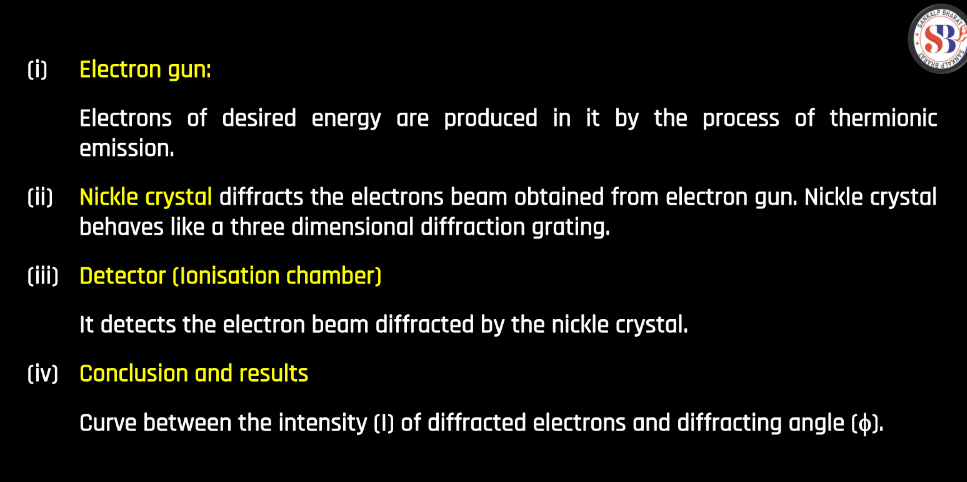
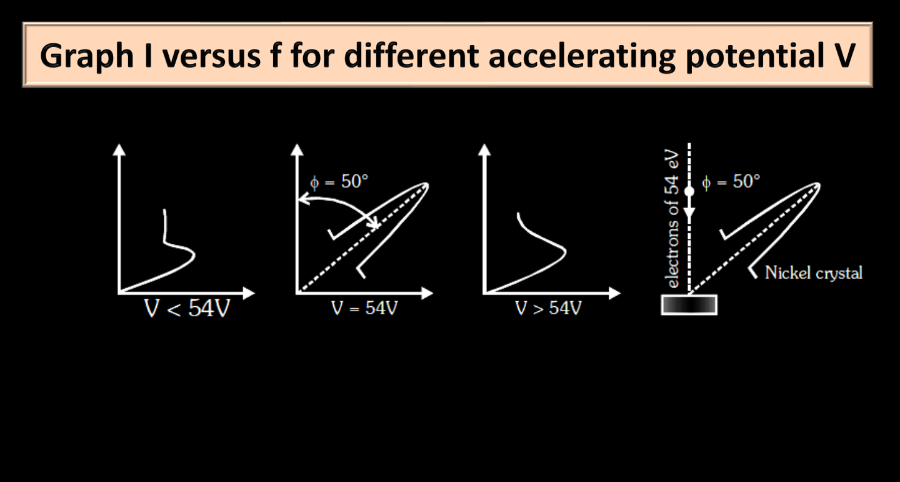
Davisson-Germer Experiment
The Davisson-Germer Experiment, conducted in 1927, demonstrated the wave-like nature of electrons. They shot electrons at a crystal surface and observed the scattered patterns. According to classical physics, particles should behave like billiard balls, but the observed patterns resembled waves. This surprising result aligned with the emerging theory of quantum mechanics. Louis de Broglie proposed that articles, including electrons, exhibit wave-particle duality. The experiment provided experimental support for this idea, confirming that electrons could display wave-like behavior. This groundbreaking insight contributed significantly to our understanding of the quantum nature of particles, setting the stage for the development of quantum mechanics.

To learn more about the Dual Nature, Photoelectric Effect, do watch the video:


 Ohm's Law: Definition, Formula, Limitati...
Ohm's Law: Definition, Formula, Limitati...
 Newton's First Law of Motion: Definition...
Newton's First Law of Motion: Definition...
 Kepler's Laws of Planetary Motion: First...
Kepler's Laws of Planetary Motion: First...













