For students preparing for the CUET Chemistry Exam, focusing on the core chemistry syllabus is beneficial. The CUET Chemistry Syllabus 2025 includes – Physical Chemistry and Organic and Inorganic Chemistry of Class 11th and 12th. Topics like thermodynamics, kinetics, equilibrium, and organic chemistry principles, including functional groups, reactions, and nomenclature, are also essential.
All the candidates appearing for the Common University Entrance Test (CUET Chemistry Exam), must practice these 100 Important Multiple Choice Questions that cover whole subjects prepared by the expert CUET Faculty. These questions are formed by analyzing the current Chemistry syllabus, exam pattern, and the previous year’s CUET Chemistry Exams. Mastering these top MCQs ensures a strong grasp of core chemistry concepts, ultimately leading to success in the CUET Exam.
CUET Chemistry Exam – Most Important MCQs with Solutions
Q1. For a substance at a given temperature, the osmotic pressure of its concentrated solution
(a) Is same as that of dilute solution
(b) Is lower than that of dilute solution
(c) Is higher than that of dilute solution
(d) Cannot be compared with osmotic pressure of dilute solution
Answer: (C)
Q2. In the electrolysis of alumina to obtain aluminum metal, cryolite is added mainly to
(a) Lower the melting point of alumina
(b) Dissolve alumina in molten cryolite
(c) Remove the impurities of alumina
(d) None of these
Answer: (A)
Q3. When Br2 is treated with an aqueous solution of NaF, NaCl, and NaI separately
(a) F2 , Cl2, I2 are liberated
(b) Only F2 and Cl2 are liberated
(c) Only Cl2 is liberated
(d) Only I2 is liberated
Answer: (D)
Q4. The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is
(a) Tertiary < secondary < primary
(b) Tertiary > secondary >primary
(c) Tertiary <secondary >primary
(d) Tertiary > secondary < primary
Answer: (B)
Q5. Raoult’s law is obeyed by each constituent of a binary liquid solution when:
(a) The forces of attraction between like molecules are greater than those between unlike molecules
(b) The forces of attraction between like molecules are smaller than those between unlike molecules
(c) The forces of attraction between like molecules are identical with those between unlike molecules
(d) The volume occupied by unlike molecules are different
Answer: (C)
Q6. In a cubic structure of compound which is made from X and Y, where X atoms are at the corners of the cube and Y at the face centers of the cube. The molecular formula of the compound is:
(a) X2 Y
(b) X3 Y
(c) XY2
(d) XY3
Answer: (D)
Q7. The product “Y” in the following reaction sequence is
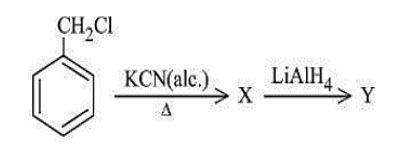
(a) C6H5-CH2NH2
(b) C6H5-CH2OH
(c) C6H5-COOH
(d) C6H5-CH2CH2NH2
Answer: (D)
Q8. Identify the true statements (s):
(A) A catalyst is chemically unchanged at the end of a reaction
(B) A catalyst may appear in the kinetic rate equation of the reaction
(C) A catalyst will not affect the composition of an equilibrium mixture
(D) A catalyst cannot cause a non-spontaneous reaction to proceed
(a) A and B only
(b) C and D only
(c) A, B, and C only
(d) A, B, C, and D all
Answer: (D)
Q9. On treating a mixture of two alkyl halides with sodium metal in dry ether, 2-methyl propane was obtained. The alkyl halides are
(a) 2-chloropropane and chloromethane
(b) 2-chloropropane and chloroethane
(c) Chloromethane and chloroethane
(d) Chloromethane and 1-chloropropane
Answer: (A)
Q10. The existence of two different coloured complexes of [Co(NH3)4Cl2]+ is due to
(a) Ionization isomerism
(b) Coordination isomerism
(c) Linkage isomerism
(d) Geometrical isomerism
Answer: (D)
Q11. When acetaldehyde is heated with Fehling’s solution, a red precipitate is formed. Which of the following is that?
(a) Cu2O
(b) Cu
(c) CuO
(d) CuSO4
Answer: (A)
Q12. CH3Br + OH → CH3OH + Br reaction proceeds by S2 mechanism. Its rate is dependent on the concentration of
(a) CHBr, OH
(b) Only CH3Br
(c) Only OH
(d) CHBr, CH₂OН
Answer: (A)
Q13. CHBr + KCN (alc.) → X Reduction/Na+ C2H5OH → Y, What is Y in the series?
(a) CHCN
(b) C2H5CN
(c) C2H5NH2
(d) CH3NH2
Answer: (C)
Q14. Which of the following compound will exhibit geometrical isomerism?
(a) 1-phenyl-2-butene
(b) 3-phenyl-1-butene
(c) 2-phenyl-1-butene
(d) 1,1-diphenyl-1-propane
Answer: (A)
Q15. The rate of a chemical reaction generally increases rapidly even with small temperature increase because of rapid increase in the:
(a) Collision frequency
(b) Fraction of molecules
(c) Activation energy
(d) Average kinetic energy of molecules
Answer: (B)
Q16. Which of the following statements is not true about colloidal solution?
(a) These are visible under a powerful microscope
(b) Their particles do not settle down with the passage of time
(c) These particles are electrically charged
(d) These are homogeneous in nature
Answer: (D)
Q17. When salicylic acid is treated with acetic anhydride we get
(a) Aspirin
(b) Paracetamol
(c) Salol
(d) None of these
Answer: (A)
Q18. Which of the following fibres are used for the formation of aircraft canopies?
(a) Nylon
(b) Dacron
(c) Orlon
(d) Terylene
Answer: (B)
Q19. Which polymer is used in non-stick cooking vessels?
(a) Polythene
(b) Isoprene
(c) Teflon
(d) Neoprene
Answer: (C)
Q20. The following compound will undergo electrophilic substitution more readily than benzene
(a) Nitrobenzene
(b) Benzoic acid
(c) Benzaldehyde
(d) Phenol
Answer: (D)
Q21. Liquation process is used for the concentration of which ore?
(a) Stibnite
(b) Tinstone
(c) Limestone
(d) Haematite
Answer: (A)
Conclusion
Students preparing for the CUET Chemistry Exam are strongly encouraged to download the Top 100 Most Questions PDF with Solutions. Additionally, practicing with these top questions enables students to identify their strengths and weaknesses, allowing for targeted study and efficient exam preparation. Don’t miss out on this opportunity to enhance your chemistry knowledge and excel in the CUET Exam—download the Top 100 Most Questions PDF today!
Top 100 Most Important CUET Chemistry Questions PDF Download Link
Get the Top 100 Most Important CUET Chemistry Questions PDF to boost your preparation. This PDF includes carefully selected questions covering key topics like organic, inorganic, and physical chemistry. Solving these questions will help you understand the exam pattern, improve accuracy, and increase confidence. It’s a great resource for last-minute revision and self-assessment. Students can easily download the PDF and start practicing right away. The direct link to download the CUET Chemistry Top 100 Most Important MCQs PDF with Solutions has been shared here for quick access.



 Best CUET Coaching in Delhi - Factors De...
Best CUET Coaching in Delhi - Factors De...
 Best CUET Coaching in Noida - Get Top 5 ...
Best CUET Coaching in Noida - Get Top 5 ...
 CUET PG Previous Year Question Papers wi...
CUET PG Previous Year Question Papers wi...












