What are Ligands?
Ligands are molecules that bind to other molecules, often larger ones like proteins. Imagine a puzzle where the ligand is a piece that perfectly fits into a specific part of the larger molecule, like a key fitting into a lock. This binding is crucial for various biological processes, like signaling in cells. In the body, ligands can be hormones, neurotransmitters, or even drugs. Their binding to proteins triggers specific responses, influencing cellular functions. Think of it as a precise interaction that controls different activities in the body, such as regulating growth growth, transmitting signals between nerve cells, or blocking certain reactions. Understanding ligands is essential in drug development, as drugs often work by mimicking or blocking the actions of natural ligands to achieve therapeutic effects.
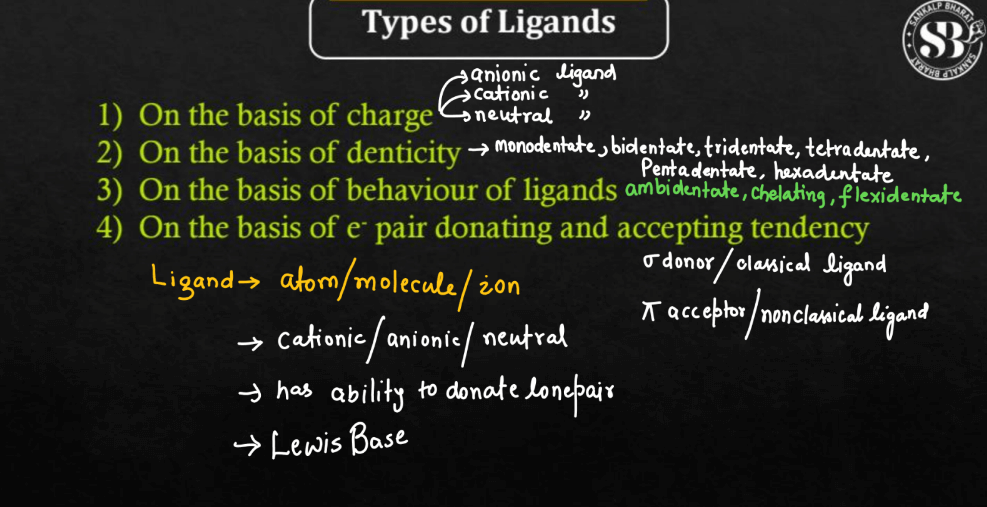
Types of Ligands
Ligands are molecules or ions that form coordination complexes with metal ions. There are various types of ligands based on their charge and coordination ability.
- On the Basis of Charge
- On the Basis of Denticity
- On the Basis of Behavior of Ligands
- On the Basis of electron pair donating and accepting tendency
On the Basis of Charge
Ligands are molecules or ions that bind to a central metal atom in coordination compounds. Based on charge, ligands can be categorized into three types namely: Anionic ligands, Cationic ligands, and Neutral Ligands. The nature of ligands influences the overall properties and reactivity of coordination compounds.
| Ligands on the Basis of Charge | |
| Types of Ligands | Description |
| Anionic Ligands | These ligands carry a negative charge, such as chloride (Cl-) or hydroxide (OH-). They denote electron pairs to the metal center. |
| Cationic Ligands | Ligands with a positive charge, like ammonium (NH4+), fall into this category. They accept electron pairs from the metal. |
| Neutral Ligands | Ligands without a net charge, like water (H2O) or ammonia (NH3), are considered neutral. They also donate electron pairs to the metal. |
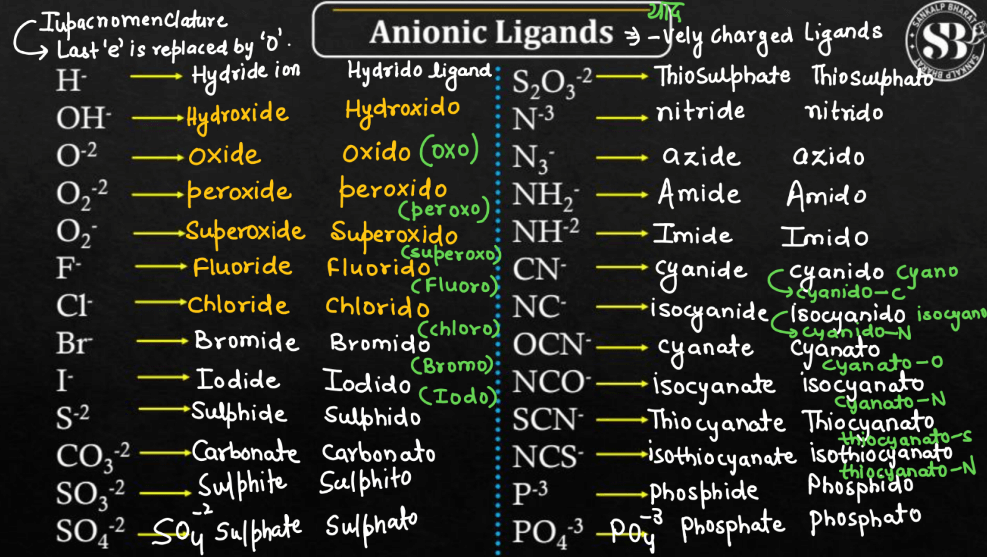
Anionic Ligands
Anionic ligands are negatively charged molecules or ions that form coordination compounds by binding to a central metal atom. These ligands play a crucial role in chemistry role in chemistry and often provide stability to metal complexes. Common examples include chloride (Cl-), sulfate (SO4^2-), and cyanide (CN-). Anionic ligands donate electron pairs to the metal center, forming coordinate covalent bonds. The metal cation attracts the negatively charged ligand, creating a balanced compound. The specific anionic ligands in a coordination complex influence the compound’s properties, such as color, magnetic behavior, and reactivity, making chemical reactions and biological processes.
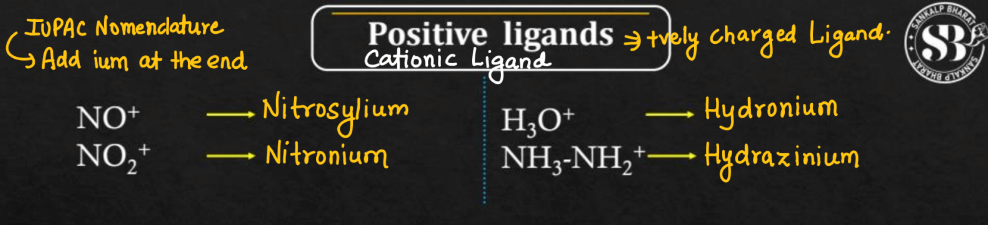
Cationic Ligands
Cationic ligands are positively charged molecules or ions that bind to a central metal atom in coordination compounds. These ligands play a significant role in forming stable complexes with metals. Examples of cationic ligands include ammonium (NH4+), which is a positively charged ion, and phosphine derivatives like triethyl phosphine (Et3P+). Cationic ligands typically donate electron pairs to the metal center, forming coordinate covalent bonds. The interaction between the positively charged ligands and the metal cation results in the overall charge neutrality of the coordination complex. The choice of cationic ligands influences the compound’s properties, reactivity, and applications in various fields, including catalysis and medicinal chemistry.
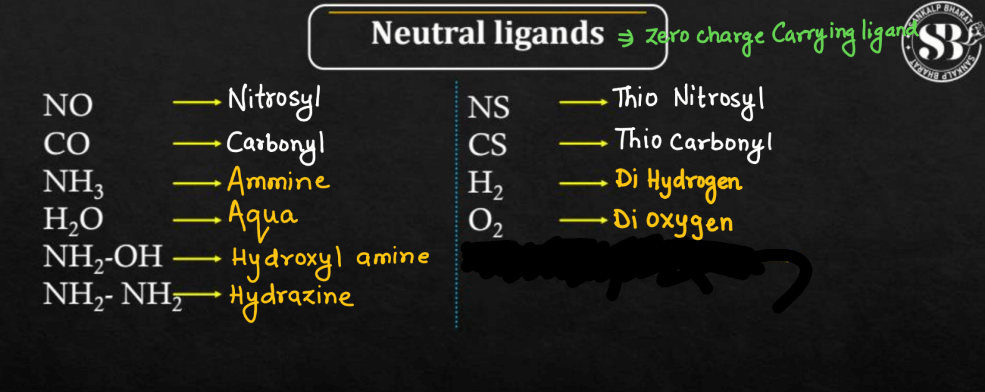
Neutral Ligands
Neutral ligands are molecules or ions that form coordination compounds by binding to a central metal atom without carrying a net positive or negative charge. Unlike anionic (negatively charged) or cationic (positively charged) ligands, neutral ligands have an equal number of protons and electrons, resulting in a neutral overall charge. Examples of neutral ligands include water (H2O), ammonia (NH3), and carbon monoxide (CO). These ligands typically form coordinate covalent bonds with the metal center by donating electron pairs. The interaction between neutral ligands and metal ions in coordination complexes plays a crucial role in determining the compound’s properties, such as color, stability, and reactivity, making them essential in various chemical reactions and industrial processes.
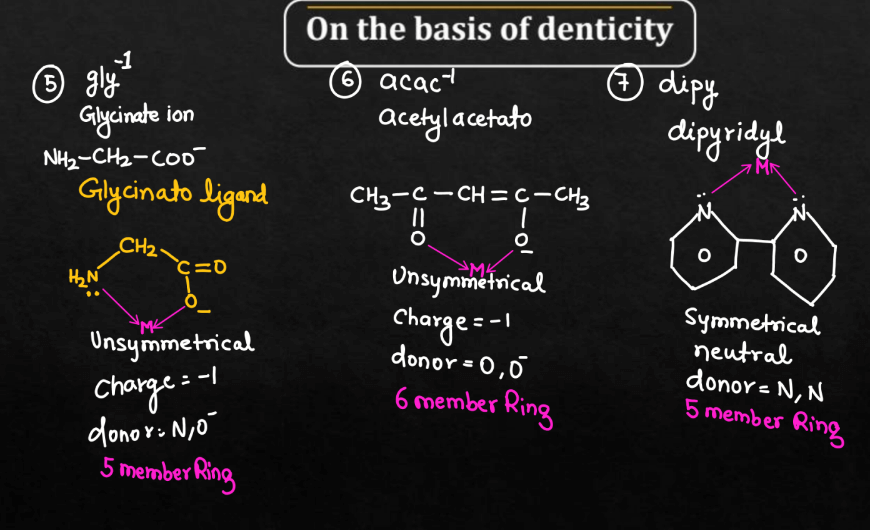
On the Basis of Denticity
Ligands can be classified based on denticity, referring to the number of donor atoms they possess. There are mainly six different types of ligands on the basis of denticity namely: Monodentate ligands, Bidentate ligands, Tridentate Ligands, Tetradentate Ligands, Pentadentate Ligands, and Hexadentate Ligands. These classifications help describe the coordination number of the metal ion in a complex.
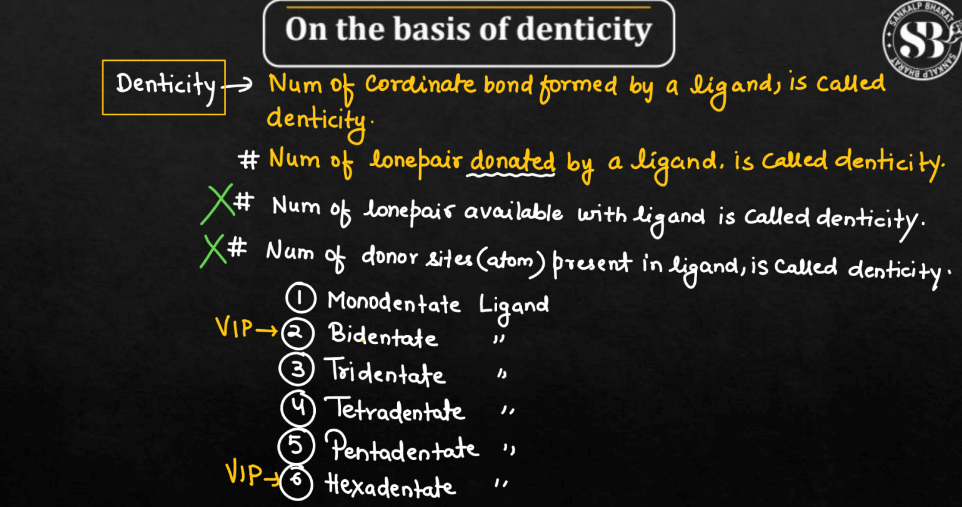
| Type of Ligands on the Basis of Denticity | |
| Types of Ligands | Description |
| Monodentate Ligands | The monodentate ligands have a single donor atom, forming one bond with the central metal ion. |
| Bidentate Ligands | The bidentate ligands are ligands with two donor atoms that can form two bonds with the metal ion. |
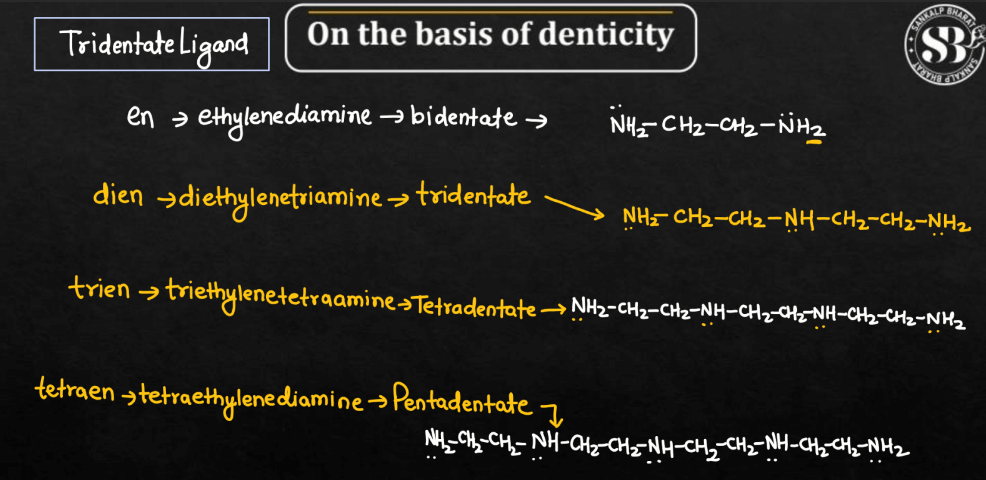
| Tridentate Ligands | Ligands with three donor atoms capable of forming three bonds with the central metal ion. |
| Tetradentate Ligands | Tetradentate ligands are ligands possessing four donor atoms, allowing them to form four bonds with the metal ion. |
| Pentadentate Ligands | Pentadentate ligands are ligands with five donor atoms, enabling them to form five bonds with the central metal ion. |
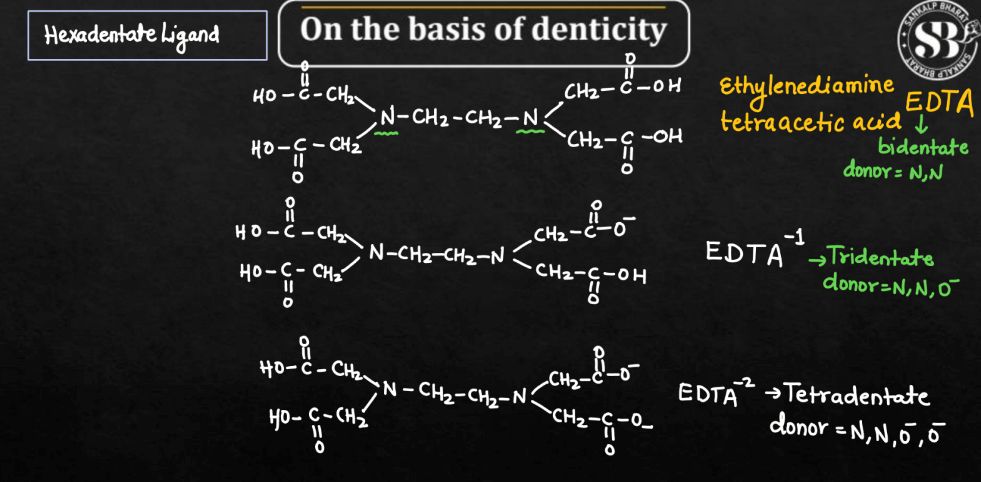
| Hexadentate Ligands | Hexadentate ligands are ligands with six donor atoms, forming six bonds with the central metal ion. EDTA (ethylenediaminetetraacetate) is a common example. |
Monodentate Ligands
Monodentate ligands are molecules that bond to a metal atom through a single atom or a small group of atoms. Imagine a metal atom as a central hub, and the monodentate ligand as a single arm reaching out to connect. This ligand forms a stable link with the metal, much like a handshake. Unlike multidentate ligands that can bind at multiple points simultaneously, monodentate ligands offer a singular connection. This simplicity in bonding makes them essential in coordination chemistry, where metal complexes play crucial roles in various chemical processes, from biological reactions to industrial catalysis. Examples include ammonia, chloride ions, and water in the coordination sphere of a metal.

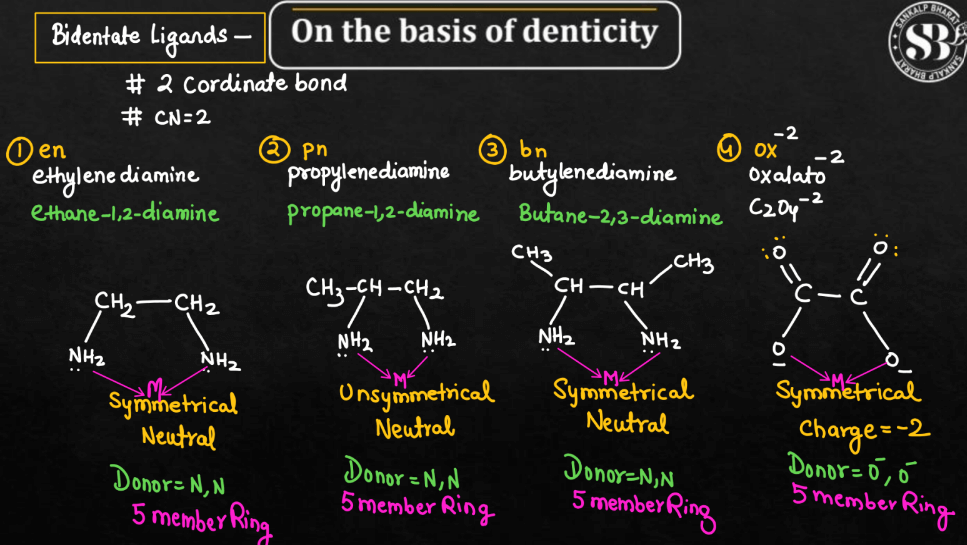
Bidentate Ligands
Bidentate ligands are molecules that bond to a metal atom using two points of attachment. Picture them as a pair of hands reaching out to grasp a metal atom. This dual bonding capability distinguishes them from monodentate ligands, enhancing the stability of the metal complexes they form. This type of ligand often creates more rigid and geometrically interesting structures in coordination compounds. Common bidentate ligands include ethylenediamine, which has two amino groups, and oxalate, which has two amino groups, and oxalate, which has two carboxylate groups. The ability of bidentate ligands to form stronger and more significant in various fields, such as medicinal chemistry and material science.
On the Basis of Behavior of Ligands
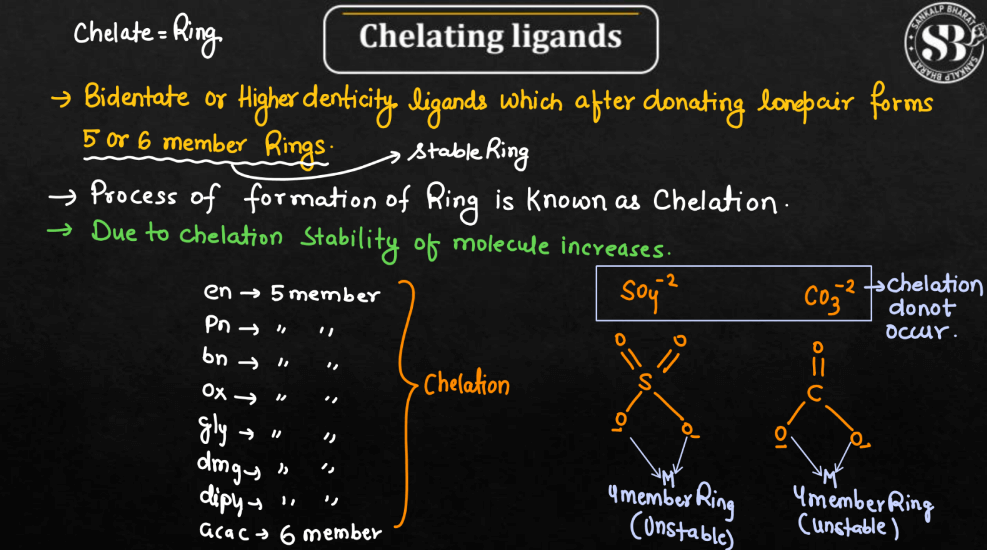
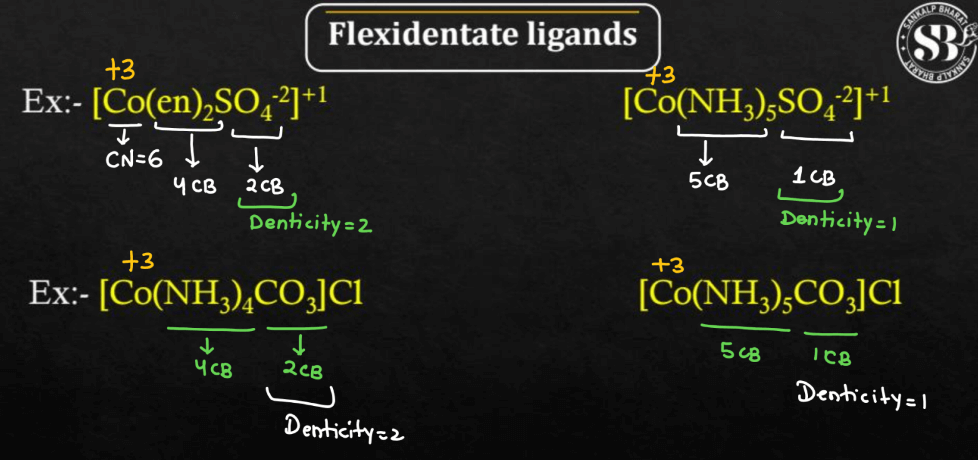
Ligands can be classified based on their behavior into three different types namely: Chelating Ligands, Ambidentate Ligands, and Flexidentate Ligands. The behavior of ligands in coordination chemistry is crucial for understanding the structure and properties of coordination compounds.

| Types of Ligands on the Basis of Behavior of Ligands | |
| Types of Ligands | Description |
| Chelating Ligands | These ligands have multiple donor atoms and can form coordination bonds at more than one site on the central metal ion. Chelating ligands are often denoted as chelates. |
| Ambidentate Ligands | Ambidentate ligands are molecules in chemistry that can bind to a metal ion at two different sites, offering flexibility in coordination. |
| Flexidentate Ligands | Flexidentate ligands are molecules in chemistry that can bind to a metal ion at multiple sites but are not necessarily restricted to a fixed number of binding sites. |
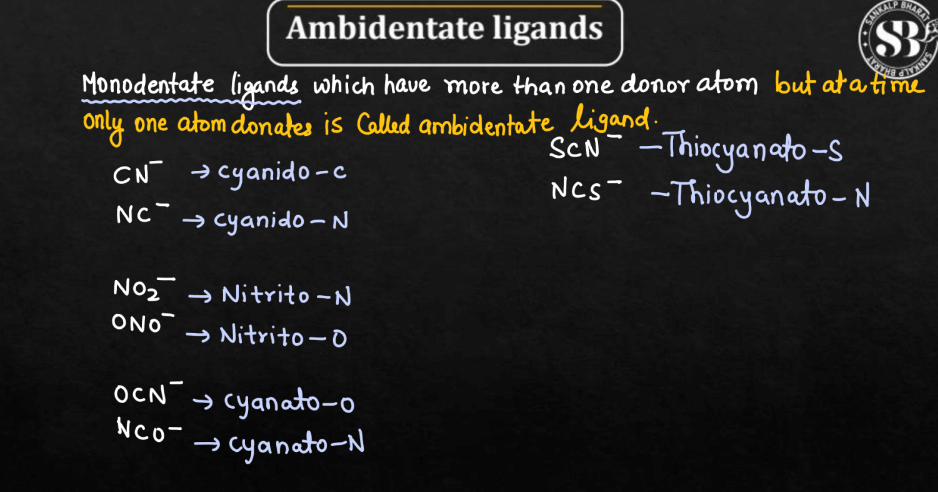
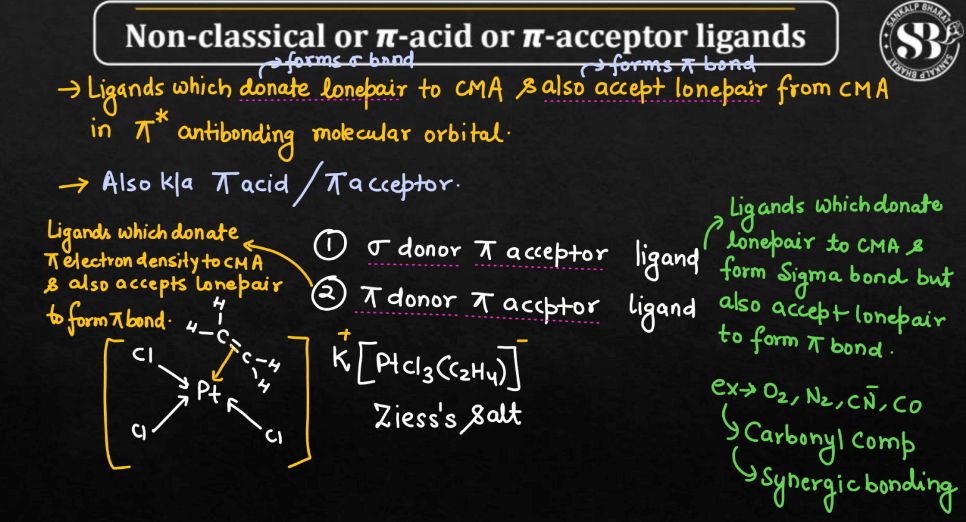
On the Basis of electron pair donating and accepting tendency
Ligands can be classified into two main types on the basis of electron pair donating and accepting tendency namely: Classical or only σ-donor Ligands and Non-classical or π-acid or π-acceptor ligands.
| Ligand on the basis of electron pair donating and accepting tendency | |
| Types of Ligands | Description |
| Classical or only σ-donor Ligands | Classical ligands are molecules that can bind to metal ions, forming coordination compounds. They typically have lone pairs of electrons, facilitating metal coordination. |
| Non-classical or π-acid or π-acceptor ligands | Non-classical ligands refer to molecules that, unlike traditional ligands, may not primarily donate lone pairs but still form coordination compounds with metal ions. |
For More Information related to Ligands, Must watch the video below:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













