Chromatography is a technique used in chemistry to separate and analyze mixtures into their individual components. Thin-layer chromatography (TLC) is a specific type of chromatography where a thin, special paper or plate is used as the stationary phase. It involves a thin, special paper or plate where the mixture is placed. When the paper is dipped in a solvent, the components of the mixture move at different rates, allowing them to be separated. Scientists use this technique to identify substances in a sample quickly and effectively. Thin-layer chromatography was developed by two scientists named Martin and Synge in the late 1930s, earning them the Nobel Prize in Chemistry in 19952.
What is Thin Layer Chromatography?
Thin Layer Chromatography (TLC) is a widely used chromatographic technique in Chemistry for separating and analyzing mixtures. Thin Layer Chromatography is relatively quick, easy, and inexpensive compared to other chromatographic techniques, making it a popular choice in laboratories for qualitative analysis and to monitor the progress of chemical reactions.
In simple terms, Thin layer chromatography is like a competition for different substances in a mixture. Imagine a special flat surface where the race happens. When you pour a liquid on it, some parts of the mixture stick to the surface, while others dissolve and move. Different parts of the mixture move at different speeds because some prefer to stick to the surface more. After the race is done, you can see which parts moved faster and which stayed behind. This process of thin-layer chromatography helps scientists figure out what’s in the mixture and how much of each substance is there.
Principle of Thin Layer Chromatography
Thin layer chromatography (TLC) is a chromatographic technique used to separate and analyze a mixture of compounds. The thin layer chromatography operates on the principle of differential migration of components in a mixture due to their varying affinities for the stationary phase which is a thin layer of adsorbent material (like silica gel or alumina), and the mobile phase which is a solvent or solvent mixture coated onto a flat support, usually a glass plate or a plastic sheet.
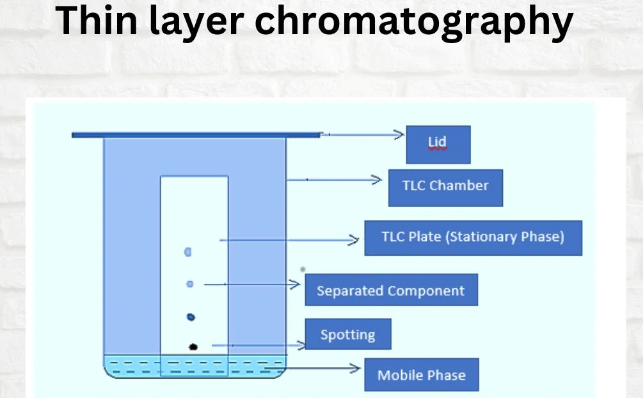
Diagram of Thin Layer Chromatography
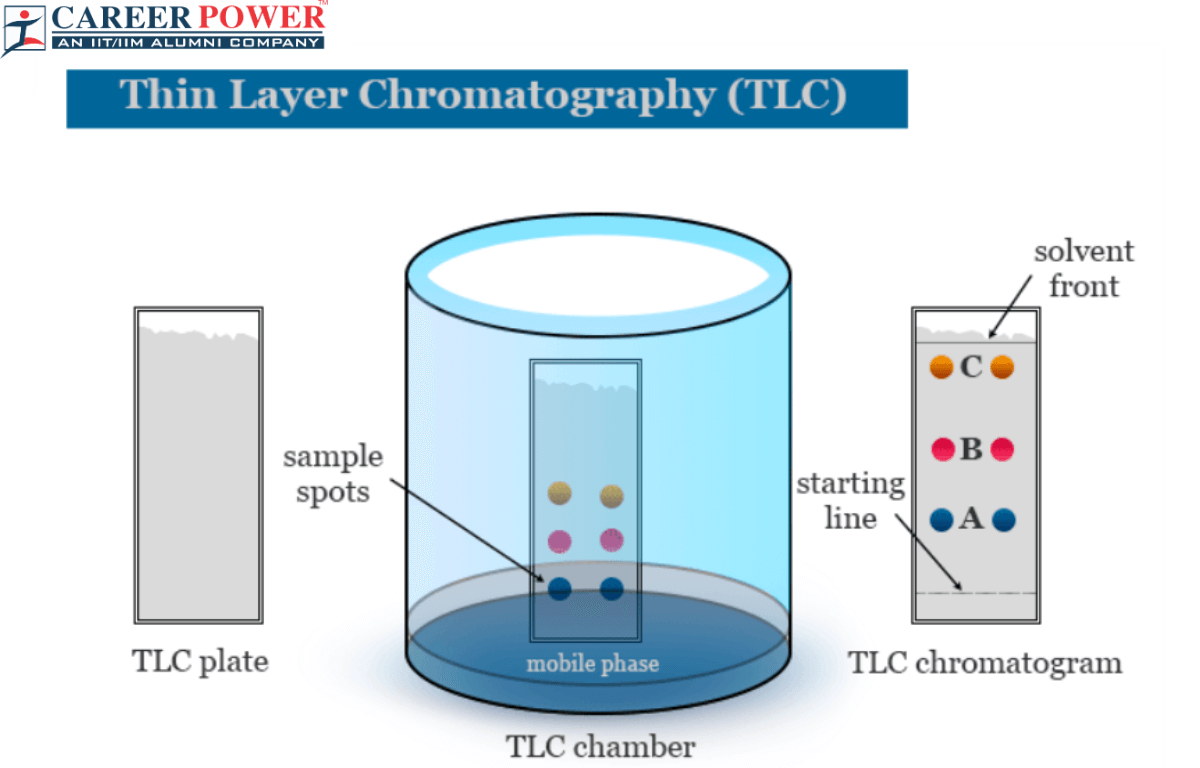
A typical thin-layer chromatography (TLC) setup includes a rectangular glass or plastic plate coated with a thin layer of adsorbent material, usually silica gel or alumina. This coated plate is the stationary phase in TLC. Near the bottom edge of the plate, a small sample spot is applied using a fine capillary tube or micropipette. The plate is then placed upright in a developing chamber containing a solvent or solvent mixture. Here we have shown a brief diagram of thin layer chromatography below.
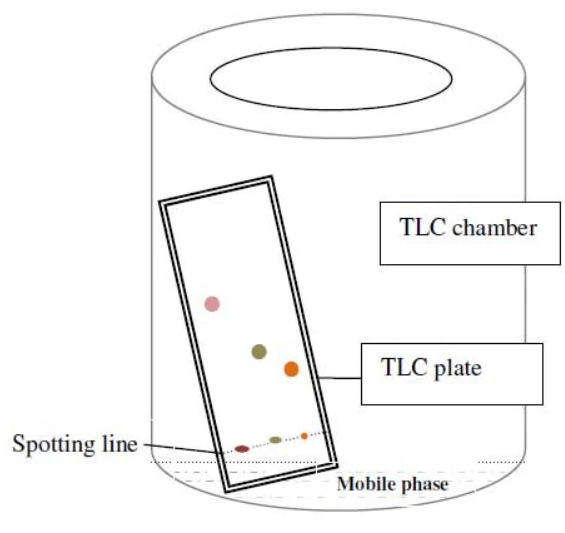
Procedure of Thing Layer Chromatography
Here we have discussed a step-by-step procedure for thin-layer chromatography. Always ensure safety precautions, such as working in a well-ventilated area and using appropriate protective gear when handling chemicals.
Materials Needed
- TLC plates (thin layer of silica gel or alumina on a glass or plastic sheet).
- Sample to be analyzed.
- Developing solvent (appropriate solvent or solvent mixture).
- Developing chamber (a closed container to hold the TLC plate and developing solvent).
- Capillary tubes or micropipettes for applying the sample.
- UV lamp or other visualization techniques (optional).
- Ruler and pencil for marking spots (optional).
Procedure for Thin Layer Chromatography
- Prepare the Thin Layer Chromatography Plate
- Mark a baseline near the bottom of the TLC plate using a pencil. This is where you’ll spot your sample.
- If necessary, activate the TLC plate by heating it in an oven before using it.
- Apply the Sample
- Using a capillary tube or micropipette, apply a small spot of your sample mixture onto the baseline. Be careful not to overload the plate, as it can affect the separation.
- Develop the Plate
- Place the TLC plate vertically in the developing chamber. Pour the developing solvent into the chamber, ensuring that the solvent level is below the baseline on the TLC plate.
- Cover the chamber to saturate the atmosphere with the solvent vapors. This ensures a consistent development process.
- Allow the solvent to move up the plate until it’s close to the top. Remove the plate from the chamber before the solvent reaches the top edge to prevent the sample from running off.
- Visualize the Separation
- After removing the plate, let it air dry to remove any remaining solvent.
- Use a UV lamp or appropriate visualization method to see the separated spots on the TLC plate. Some compounds might be invisible under normal light but visible under UV light.
- Analysis
- Measure the distance traveled by each spot from the baseline and calculate the Rf (retention factor) value for each spot. Rf = Distance traveled by compound / Distance traveled by solvent.
- Compare the Rf values of the sample components with known standards or literature values to identify the substances present in your sample.
- Clean Up
- If needed, mark the separated spots, cut them out, and extract the compounds for further analysis.
Applications of Thin Layer Chromatography
Thin Layer Chromatography (TLC) is a versatile technique used in various fields for qualitative and quantitative analysis of compounds. Thin-layer chromatography is popular due to its simplicity, speed, and cost-effectiveness, making it a valuable tool for analytical chemists and researchers in various fields. Here are some common applications of TLC:
- Drug Analysis: TLC is used in pharmaceutical industries to analyze drug purity and identify the presence of impurities in drugs and pharmaceutical products.
- Forensic Science: TLC is employed to analyze substances found at crime scenes, and identify drugs, toxins, or other chemicals in forensic samples.
- Food and Beverage Industry: It is used to analyze food additives, preservatives, and colorants. It can also be used to detect pesticide residue in food products.
- Environmental Testing: TLC helps in the analysis of environmental samples to detect pollutants and contaminants in soil, water, and air samples.
- Biochemistry: TLC is used to separate and identify amino acids, peptides, and other biomolecules.
- Education Purpose: TLC is widely used in educational labs to demonstrate the principles of chromatography and train students in analytical techniques.
- Clinical Chemistry: In clinical labs, TLC is used for analyzing blood lipids, hormones, drugs, and other compounds in body fluids.
Disadvantages of Thin Layer Chromatography
While Thin Layer Chromatography (TLC) is a widely used and valuable analytical technique, it does have some disadvantages. But despite those limitations, TLC remains a valuable tool in many applications, especially when quick qualitative analysis is needed or as a preliminary step in compound separation and identification processes.
- Limited Sensitivity: TLC is not as sensitive as some other chromatographic techniques. It may not be suitable for detecting trace amounts of compounds in a sample.
- Lack of Quantitative Data: TLC is generally more qualitative than quantitative. While it can provide information about the presence or absence of compounds, accurately quantifying the amounts can be challenging without sophisticated equipment.
- Limited Resources: The resolution (ability to separate closely related compounds) of TLC is lower compared to other chromatographic methods such as high-performance liquid chromatography (HPLC) or gas chromatography (GC). This limits its applicability in complex samples.
- Limited Mobile Phase Options: The choice of mobile phase in TLC is crucial for the separation. However, the range of solvents that can be used is limited compared to other chromatographic techniques, potentially restricting the separation of certain compounds.
- Not Suitable For High Molecular Weight Compounds: TLC is more suitable for smaller molecules. Large or high molecular weight compounds might not migrate well on the TLC plate, leading to poor separation.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













