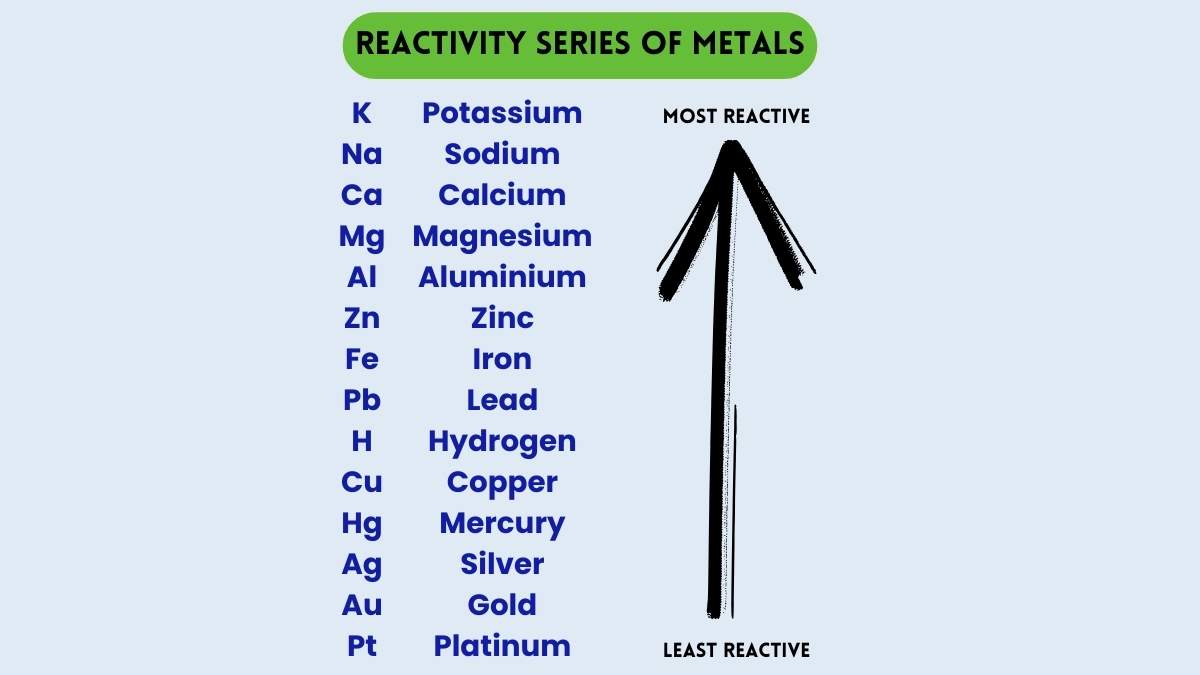
The Reactivity Series is the representation of metals based on their reactivity in descending order. Since the metals are presented from highest to lowest in the reactivity manner, it is defined as the series of metals in order of reactivity or the activity series. The reactivity of a metal mainly depends on the outer orbitals or the electronic configuration. The metals with the highest number of atoms are most reactive in nature and they tend to lose electrons easily as their electrons are far from the positively charged nucleus. The periodic table is composed of a total 95 elements that possess a metallic nature.
Reactivity Series
The table lists metals based on their reactivity. Potassium and sodium are at the top, meaning they are very reactive, while gold and platinum are at the bottom, making them the least reactive. Because they are less reactive, gold and platinum do not easily corrode, which is why they remain shiny and intact over time. In contrast, metals like zinc, aluminum, magnesium, and calcium are more reactive and can rust or corrode more quickly when exposed to the environment.
| Reactivity Series of Metals | ||
| Name of element | Symbol of element | Reactivity |
| Potassium | K | Most reactive |
| Sodium | Na | ⇑ Reactivity Increases
⇓ Reactivity Decreases |
| Calcium | Ca | |
| Magnesium | Mg | |
| Aluminium | Al | |
| Zinc | Zn | |
| Iron | Fe | |
| Lead | Pb | |
| Hydrogen | H | |
| Copper | Cu | |
| Mercury | Hg | |
| Silver | Ag | |
| Gold | Au | |
| Platinum | Pt | Least reactive |
Reactivity Series of Metals Chart
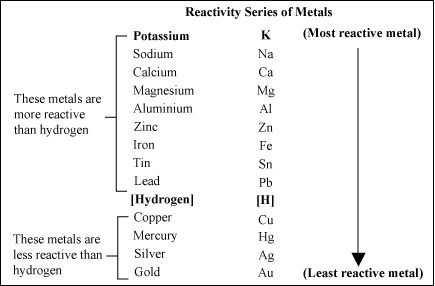
Features of Reactivity Series of Metals
- The metals at the top of the reactivity series are highly reactive therefore they are easily oxidized. This property of highly reactive metals makes them easily corrosive, for example, sodium due to its highly corrosive nature explodes when comes in contact with the air or water therefore is kept in kerosene to ensure that they are kept away from the air and moisture
- While moving downwards in the reactivity series the reactive nature of the metals reduces as it’s hard for the elements to lose electrons
- All the metals that are found above hydrogen in the reactivity series liberate H2 gas and form salt on reaction with the HCl or dilute H2SO4
- The metals ranking higher in the reactivity series require a higher amount of energy for their isolation from other compounds
- While moving down the reactivity series the electron-donating ability of the metals reduces
Reactivity Series and Corresponding Ions
The table below replicates the reactivity series of metals along with their corresponding ions and their reacting agents. Check the table below to note down the ions formed from every metal while reacting with their reacting agents.
| Reactivity Series and Corresponding Ions | ||
| Reactivity Series of Metals | Ions Formed | Reacting Agents |
| Cesium | Cs+ |
Cold Water
|
| Francium | Fr+ | |
| Rubidium | Rb+ | |
| Potassium | K+ | |
| Sodium | Na+ | |
| Lithium | Li+ | |
| Barium | Ba2+ | |
| Radium | Ra2+ | |
| Strontium | Sr2+ | |
| Calcium | Ca2+ | |
| Magnesium | Mg2+ |
Not react with cold water but highly reactive with Acids
|
| Beryllium | Be2+ | |
| Aluminium | Al3+ | |
| Titanium | Ti4+ | |
| Manganese | Mn2+ | |
| Zinc | Zn2+ | |
| Chromium | Cr3+ | |
| Iron | Fe3+ | |
| Cadmium | Cd2+ | |
| Cobalt | Co2+ | |
| Nickel | Ni2+ | |
| Tin | Sn2+ | |
| Lead | Pb2+ | |
| Hydrogen |
H+ (Non-Metal, Reference for Comparison)
|
|
| Antimony | Sb3+ |
Oxidizing Acids
|
| Bismuth | Bi3+ | |
| Copper | Cu2+ | |
| Tungsten | W3+ | |
| Mercury | Hg2+ | |
| Silver | Ag+ | |
| Platinum | Pt4+ | |
| Gold | Au3+ | |
Importance of Reactivity Series of Metals
The reactivity series is of greater importance as it replicates the study of the properties and reactivity of metals. Apart from the understanding of reactivity of metals we also get to understand multiple other factors like the results of reactions between various metals and acids, metals, and water, and the results of many other reactions can also be predicted after having a proper understanding of the Activity series of metals.
Reaction between Metals and Water
Highly reactive metals like calcium and other metals that are more reactive in nature can react with cold water and corresponding hydroxide while liberating hydrogen gas. For example, in the reaction of potassium and water, the outcome is potassium hydroxide and H2 gas as represented below:
2K + 2H2O → 2KOH + H2
Reactions between Metals and Acids
Metals like lead and other metals ranking above lead in the reactivity series form salt when reacted with sulphuric acid or hydrochloric acid. Along with the formation of salt the reaction also liberates hydrogen gas. The reaction between zinc and sulphuric acid is a great example of a reaction between metals and acids. Check the reaction below:
Zn + H2SO4 → ZnSO4 + H2
Single Displacement Reaction between Metals
High-ranking metals easily reduce the ions of low-ranking metals. Therefore whenever a single displacement reaction takes place the low-ranking metal is easily displaced by the high-ranking metal. An example of a single displacement reaction between metals is the reaction of copper from copper sulfate by zinc. Check the reaction below:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The Single displacement reaction is used on a daily basis as in thermite welding where aluminum displaces iron from its oxide or in steel making where carbon displaces iron from its oxide.
What is the shortcut for learning the reactivity series?
Memorizing the reactivity series is one of the most important and crucial parts of understanding its use and importance. In chemistry, there are multiple series and tables that have some tricks to remember them, like some used for remembering the periodic table. Similarly, to remember the Reactivity Series there are numerous tricks like:
- “Please Stop Calling Me A Zebra, I Like Cute Silly Goofy Penguins” where the first character of every word represents the first character of every metal in the reactivity series
|
Learning Reactivity Series of Metals by Song
|
|
| Elements of Reactivity Series |
Sentence to Remember Reactivity Series
|
| Potassium (K) | Please |
| Sodium (Na) | Stop |
| Calcium (Ca) | Calling |
| Magnesium (Mg) | Me |
| Aluminium (Al) | A |
| Zinc (Zn) | Zebra, |
| Iron (Fe) | I |
| Lead (Pb) | Like |
| Copper (Cu) | Cute |
| Silver (Ag) | Silly |
| Gold (Au) | Goofy |
| Platinium (Pt) | Penguins |
- “Please send cats, monkeys, and zebras in lovely happy cages made of silver, gold & platinum.” In this mnemonic, every first character of the words denotes the first character of every metal thus making it easier to remember the reactivity series.
|
Reactivity Series
|
|
| Elements of Reactivity Series |
Sentence to Remember Reactivity Series
|
| Potassium (K) | Please |
| Sodium (Na) | send |
| Calcium (Ca) | cats, |
| Magnesium (Mg) | monkeys |
| Aluminium (Al) | and |
| Zinc (Zn) | zebras |
| Iron (Fe) | in |
| Lead (Pb) | lovely |
| Hydrogen (H) | happy |
| Copper (Cu) | cages |
| Mercury (Hg) | made of |
| Silver (Ag) | silver, |
| Gold (Au) | gold & |
| Platinium (Pt) | platinum |
The representation of metals in descending series is known as the Reactivity Series.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













