What are Coordinate Compounds?
Coordinate compounds, also known as coordination compounds, are molecules composed of a central metal atom or ion bonded to surrounding molecules or ions called ligands. Think of the central metal as the “host” and the ligands as “guests” attached to it. What makes these compounds interesting is the formation of coordinate bonds, where the metal and ligands share pairs of electrons. In simpler terms, it’s like a party where the metal is the host and the ligands are guests holding hands with the host.
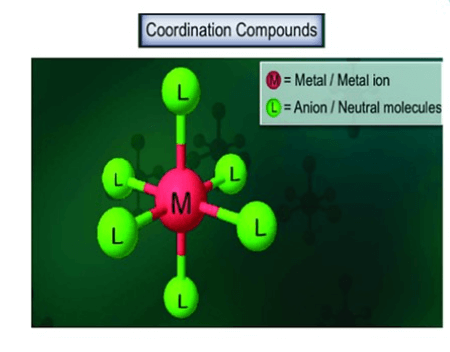 The shared hand-holding represents the coordinate bond. This unique bonding results in a stable structure with distinct properties, often different from the individual components. For example, in a complex like “[Co(NH3)6]3+“, cobalt (Co) is the host, and six ammonia (NH3) molecules are guests forming coordinate bonds. Understanding coordinate compounds is crucial in fields like chemistry and biochemistry, explaining the diverse behaviors of certain substances.
The shared hand-holding represents the coordinate bond. This unique bonding results in a stable structure with distinct properties, often different from the individual components. For example, in a complex like “[Co(NH3)6]3+“, cobalt (Co) is the host, and six ammonia (NH3) molecules are guests forming coordinate bonds. Understanding coordinate compounds is crucial in fields like chemistry and biochemistry, explaining the diverse behaviors of certain substances.
IUPAC Nomenclature of Coordination Compounds
In IUPAC Nomenclature, coordination compounds are named systematically to convey information about their composition. Start with the cation, typically a metal, followed by the ligands (molecules or ions bonded to the metal). For ligands, use prefixes like “di-” or “tri-” to indicate the number of ligands, and end names with “o” when ligand names finish in “a”. Arrange ligands alphabetically, considering their names as a whole. Indicate the metal’s oxidation state using Roman numerals in parentheses after the metal name. If there’s a negatively charged complex, use the suffix “-ate”. If multiple ligands of the same type are present, use prefixes like “bis-” or “tris-” before the ligand name. This systematic approach ensures clarity and consistency when naming coordination compounds.
Rules of Naming Coordination Compounds
Here we have discussed a few points that will highlight the basic rules for naming coordination compounds. Remember that practice is crucial for mastering the naming of coordination compounds, as it involves understanding the structure of complex ions and their nomenclature.
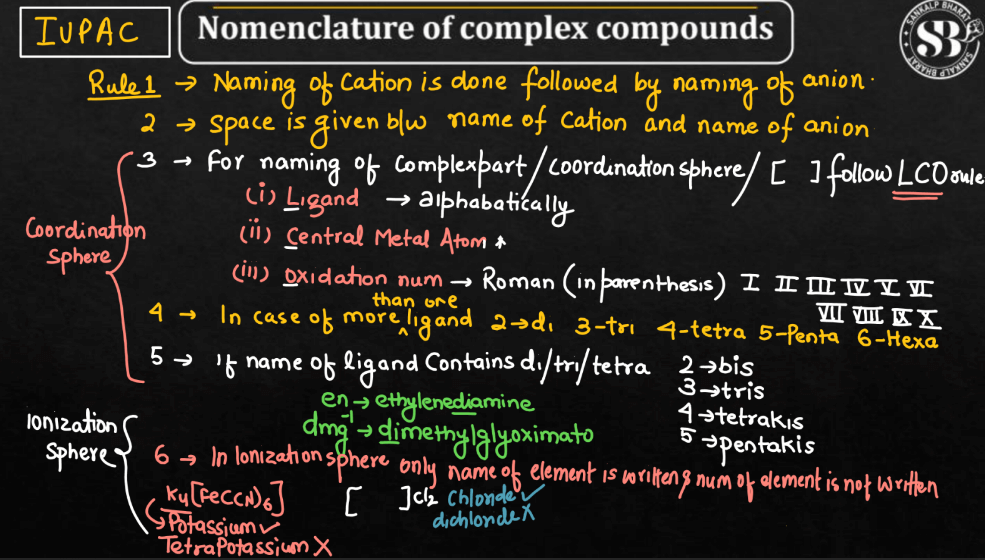
Rule 1: Cation before Anion: In the chemical formula of a coordination compound, the cation (usually a metal) is named before the anion (usually a complex ion).
Rule 2: Name of the Ligands: Lingands are named before the metal or ion. Ligands are named in alphabetical order, and prefixes indicating the number of each type of ligand are used (di-, tri-, tetra-, etc.).
Rule 3: Greek Prefixes for Ligands: When the ligand name itself has a Greek prefix (e.g., ethylenediamine), this prefix is not considered when alphabetizing. The Greek prefixes di-, tri-, etc., indicating the number of ligands, are used instead.
Rule 4: Coordination Number: The coordination number of the metal ion is indicated by the Roman numeral in parentheses after the metal name. It represents the number of donor atoms attached to the central metal ion.
Rule 5: Oxidation State of the Metal: The oxidation state of the metal is indicated by a Roman numeral in parentheses after the metal name.
Rule 6: Anionic Ligands: If the complex ion is negatively charged, the ending of the metal’s name is modified to “-ate”. For example, “chloro” becomes “chlorate”.
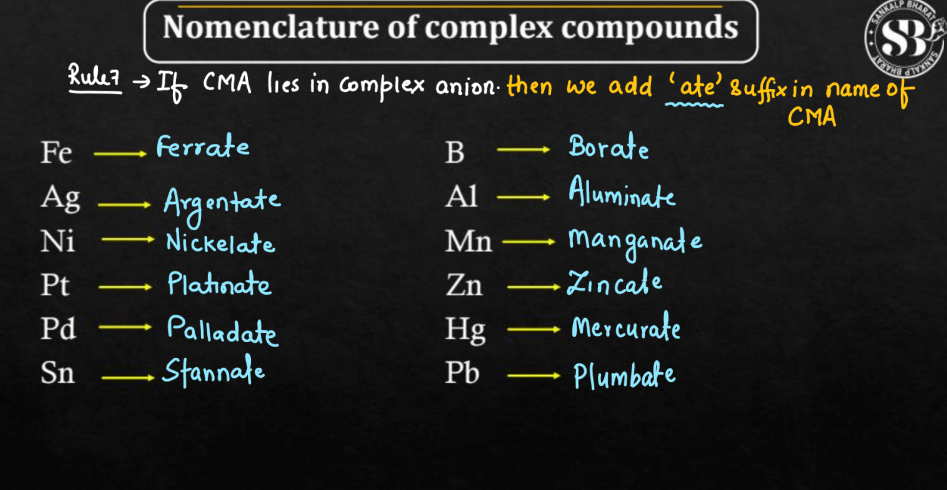
Rule 7: Neutral Ligands: For neutral ligands, the name is the same as the molecule’s name.
Rule 8: Different isomers of coordination compounds may exist, and they should be named accordingly. Structural isomers, geometric isomers, and optical isomers are considered.
Some Examples of Naming Coordinate Compounds
Here we have discussed a few examples that will help the students to clearly understand the naming of coordination compounds. These examples follow the IUPAC naming conventions for coordination compounds.
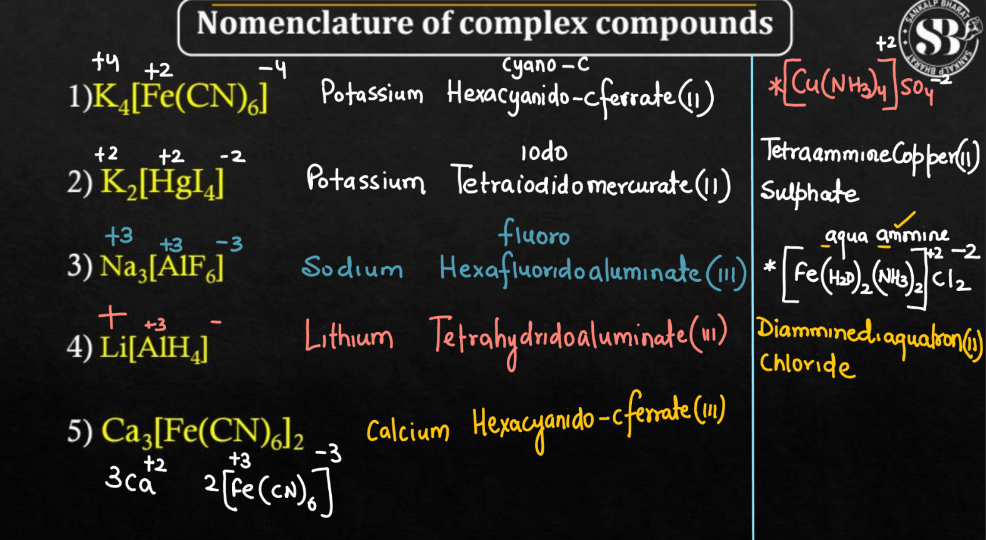
- [Co(NH3)4Cl2]Cl – Tetraamminechloridocobalt (III) chloride
- [Fe(CN)6]3– – Hexacyanidoferrate (III) ion
- [Pt(NH3)2Cl2] – Diamminedichlooridoplatinum (II)
- [Cu(en)2]2+ – Bisethylenediaminecopper (II) ion
- [Cr(H2O)6]3+ – Hexaaquachromium (III) ion
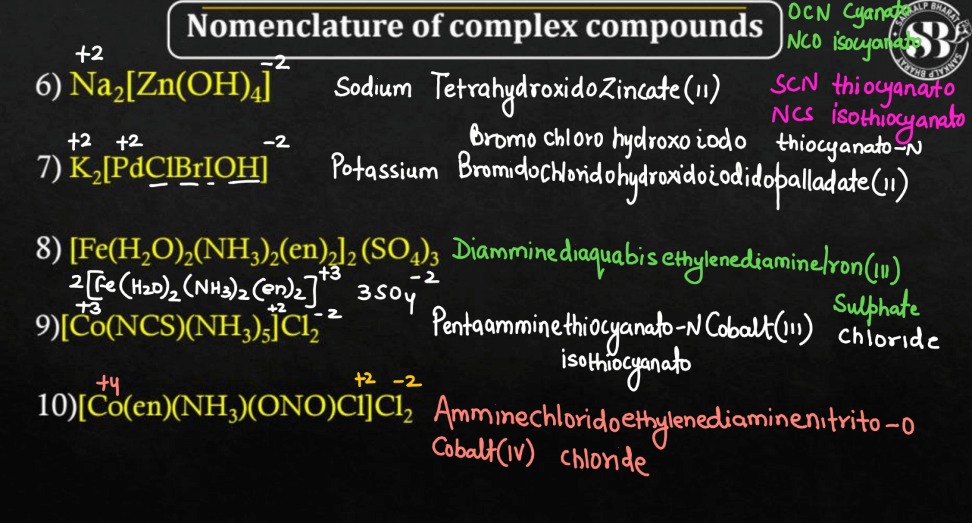
- [Ni(CO)4] – Tetracarbonylnylnickel (O)
- [Co(en)3]3+ – Trisethylenediaminecobalt (III) ion
- [PtCl4]2- – Tetrachloridoplatinate (II) ion
- [Cr(CO)6] – Hexacarbonylchromium (O)
- [Co(NH3)5Cl]Cl2 – Pentaamminechloridocobalt (III) chloride
MCQs Based on the Nomenclature of Coordinate Compound
Ques 1: The name of the complex ion [Fe(CN)6]3– is
(a) Tricyanoferrate (III) ion
(b) Hexacyanoferrate (III) ion
(c) Hexacyanoiron (III) ion
(d) Hexacyanitoferrate (III) ion
Ques 2: IUPAC name of [Pt(NH3)3(Br)(NO2)Cl]Cl is
(a) Triamminebromochloronitroplatinum (IV) chloride
(b) Triamminebromonitrochloroplatinum (IV) chloride
(c) Triamminechlorobromonitroplatinum (IV) chloride
(D) Triamminenitrochlorobromoplatinum (IV) chloride
Ques 3: The IUPAC name of complex – [Ag(H2O)2][Ag(CN)2] is
(a) Diaquasilver (II) dicyanidoargentate (II)
(b) Dicyanidosilver (I) diaquaargentate (I)
(c) Diaquasilver (I) dicyanidoargentate (I)
(d) Dicyanidosilver (II) diaquaargentate (II)
Ques 4: The IUPAC name of the complex – [Co(H2O)4(NH3)2]Cl3 is
(a) Tetraaquadiaminecobalt (II) chloride
(b) Tetraaquadiamminecobalt (III) chloride
(c) Diaminetetraaquacobalt (III) chloride
(d) Diamminetetraaquacobalt (III) chloride


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













