Define Hydration
Hydration refers to the process of adding water molecules to a substance. This can happen in various ways, such as when an ionic compound dissolves in water and water molecules surround and interact with the ions. In organic chemistry, hydration can also refer to adding water across a double bond in a chemical reaction, like in the hydration of alkenes to form alcohols. This process often involves catalysts or specific reaction conditions to facilitate the addition of water molecules. Overall, hydration involves the incorporation of water molecules into a substance, leading to changes in its properties or chemical structure, and it plays a crucial role in many chemical reactions and biological processes.
What is Hydration Energy?
Hydration energy is the energy released or absorbed when ions in a gaseous state dissolve in water to form hydrated ions. Imagine ions as tiny particles with positive or negative charges. When these ions come into contact with water molecules, they attract and surround themselves with water molecules due to their charges. This process releases energy, known as hydration energy if the attraction between the ion and water molecules is strong. Conversely, if the attraction is weak, it may absorb energy instead. Hydration energy plays a crucial role in various chemical reactions and influences the solubility and behavior of substances in water, affecting biological and environmental processes.
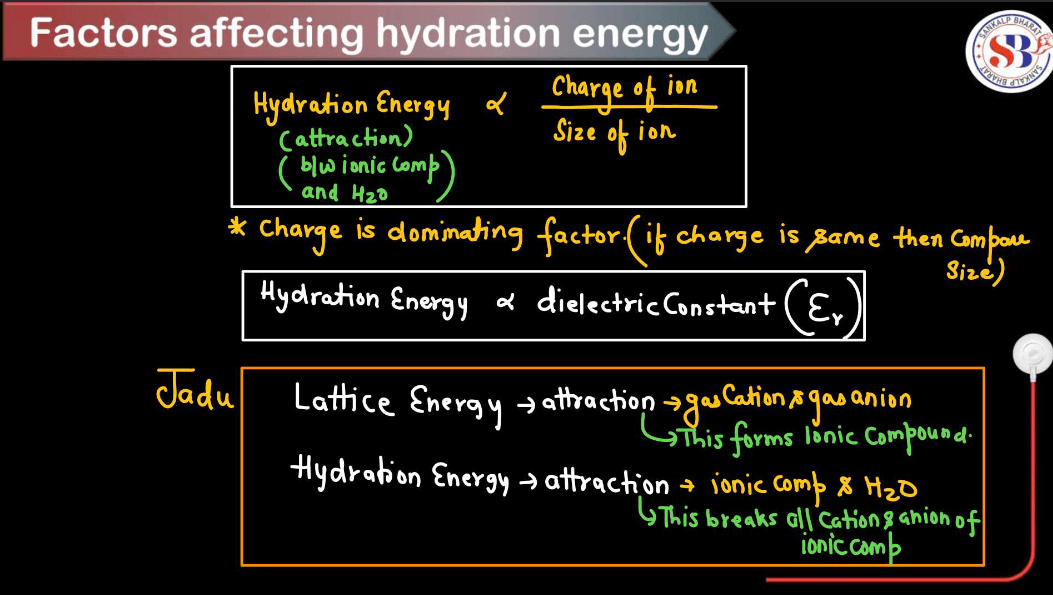
Factors Affecting Hydration Energy
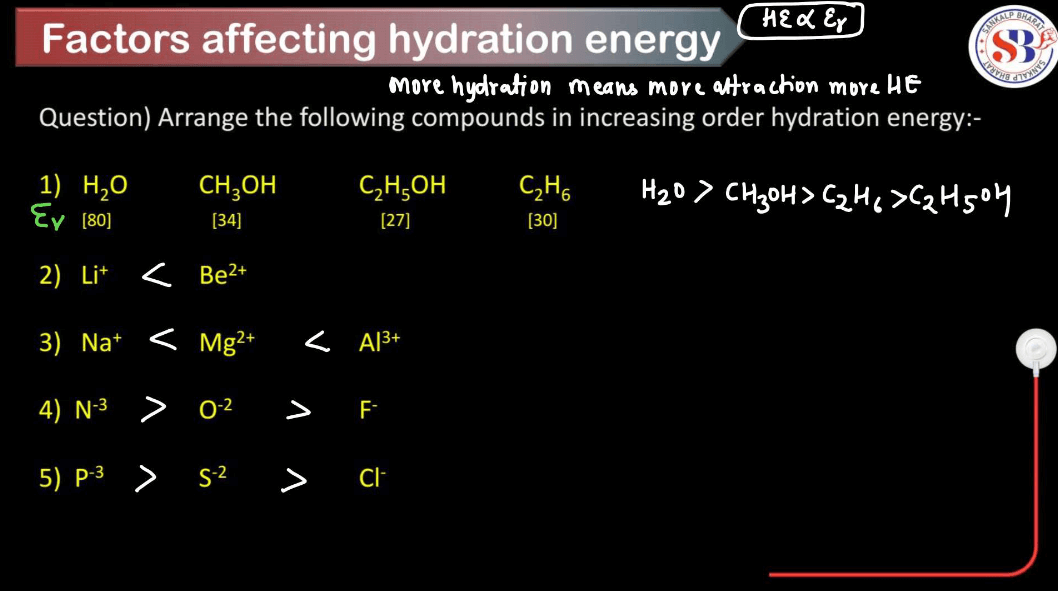
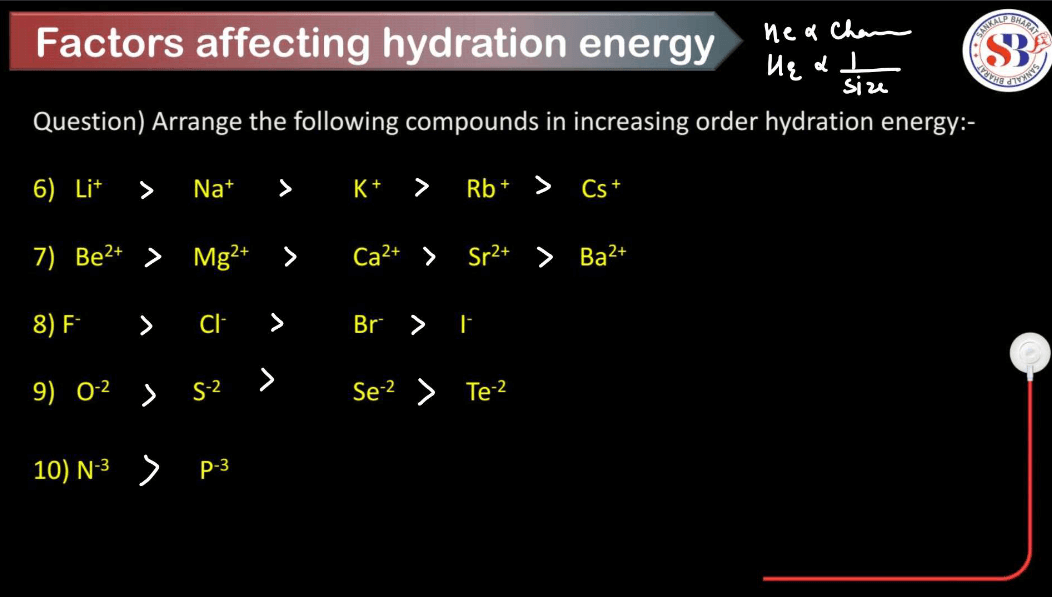
Hydration energy refers to the energy released or absorbed when ions in the gas phase combine with water molecules to form hydrated ions in solution. Several factors influence hydration energy. These factors collectively determine the strength of the ion-water interactions and, consequently, the magnitude of hydration energy for a given ion.
- Ion Size:
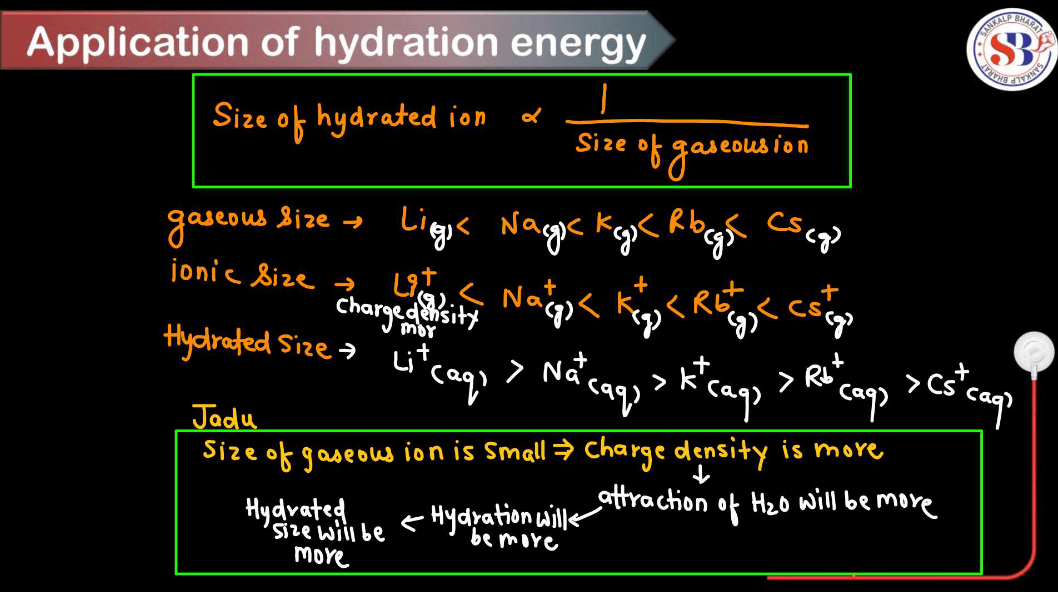
Smaller ions tend to have higher hydration energies because they can pack more water molecules around them due to their higher charge density. Larger ions have lower charge density and can’t attract water molecules as closely, resulting in lower hydration energies.
- Ion Charge:
Higher charges on ions lead to stronger electrostatic attractions between the ion and water molecules, resulting in higher hydration energies. For example, a highly charged ion like Al3+ will have a higher hydration energy compared to Na+ because it can attract more water molecules.
- Ion Polarizabilities:
Some ions can distort the electron clouds of water molecules more easily, leading to stronger interactions and higher hydration energies. For instance, larger ions or ions with more diffuse electron clouds exhibit higher polarizability and can have higher hydration energies.
- Solvent Properties:
The nature of the solvent (water in this case) can affect hydration energy. Water molecules can orient themselves in specific ways around ions to maximize electrostatic interactions, impacting hydration energies.
- Temperature:
Hydration energies are influenced by temperature. Generally, higher temperatures lead to weaker hydration energies because increased thermal energy disrupts the ion-water interactions.
Application of Hydration Energy
Hydration Energy plays a crucial role in various chemical and biological processes. Here are some applications of hydration energy.
- Solubility and Dissolution: Hydration energy affects the solubility of substances in water. Substances with higher hydration energies tend to be more soluble because the energy released during hydration compensates for the energy required to break the solute-solvent interactions.
- Ionic Reactions: In ionic reactions, hydration energy influences the stability and reactivity of ions in solution. For example, highly hydrated ions like Li+ and Na+ are more stable due to their strong hydration shells, affecting their mobility and behavior in aqueous solutions.
- Enzymatic Activity: Enzymes often work in aqueous environments where hydration energy is crucial. Hydration of substrates and active sites can influence enzyme-substrate interactions, affecting the rate and efficiency of enzymatic reactions.
- Electrolyte Solutions: Hydration energy plays a role in the conductivity of electrolyte solutions. Highly hydrated ions contribute to the conductivity of the solution by facilitating the movement of charge carriers (ions) through the medium.
- Biological Processes: In a biological system, hydration energy is important for the stability and functioning of biomolecules like proteins and nucleic acids. Water molecules form hydration shells around biomolecules, influencing their structure, stability, and interactions with other molecules.
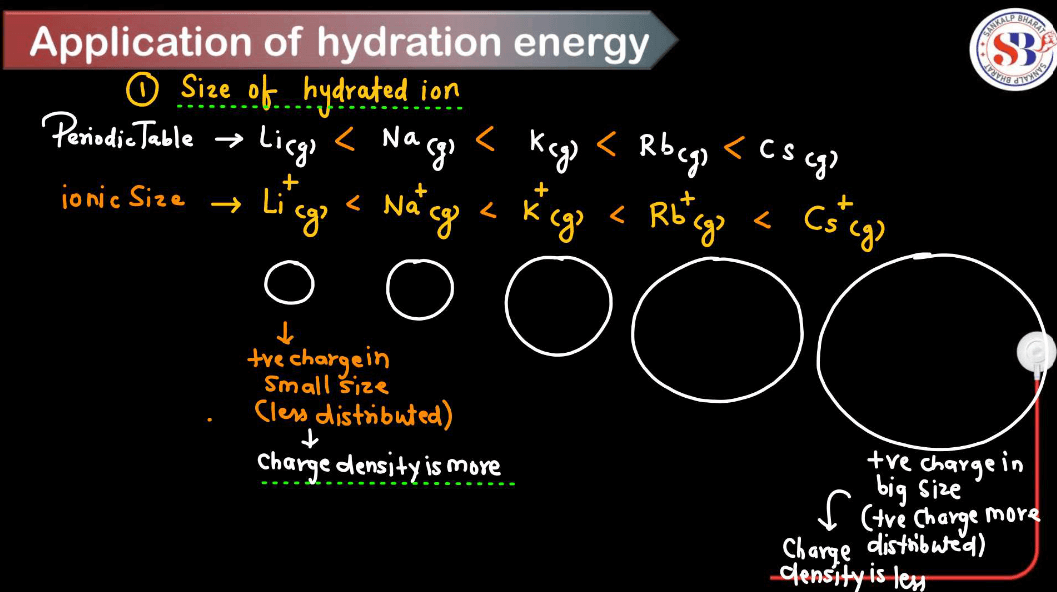


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













