Define Electrochemistry
Electrochemistry is a branch of chemistry that explores the relationship between electricity and chemical reactions. It delves into how electron movement, typically through a conductive material like a metal or an electrolyte, influences chemical transformations. At its core, electrochemistry involves two main processes: Oxidation and reduction. During oxidation, a substance loses electrons, while in reduction, another substance gains electrons. These simultaneous redox reactions occur at electrodes, which are conductive surfaces immersed in a solution containing ions.
The movement of electrons generates an electric current, and this flow of electric current, and this flow of electricity can be harnessed for various applications, including batteries, electrolysis, and corrosion prevention. Understanding electrochemical principles is crucial in designing efficient energy storage systems, powering electronic devices, and even in biological processes like nerve impulses. In essence, electrochemistry unravels the intricate dance between electrons and molecules, shaping the way we harness and utilize energy in diverse fields.
Kohlrausch’s Law
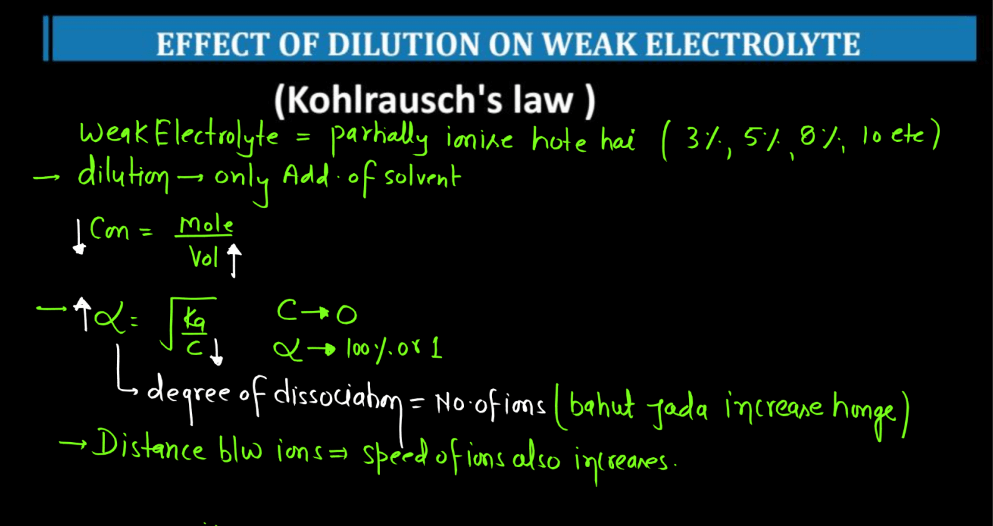
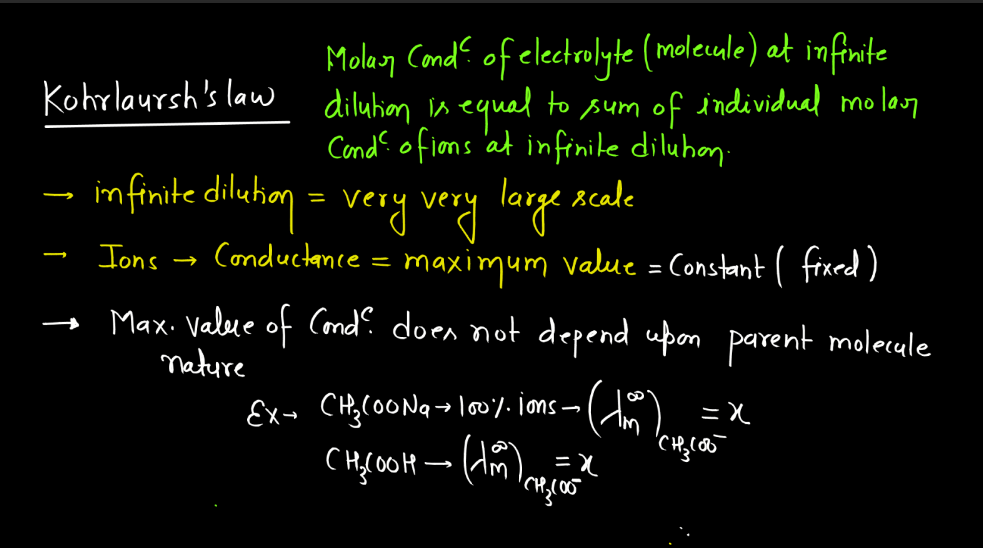
Kohlrausch’s Law, named after German physicist Friedrich Kohlrausch, relates to the conductivity of electrolyte solutions. In simple terms, it states that the molar conductivity (the ability to carry an electric current) of an electrolyte is the sum of the individual molar conductivities of its ions. When an ionic compound dissolves in water, it breaks into positive and negative ions, which conduct electricity. Kohlrausch observed that the total conductivity depends on the number of ions produced during dissociation.
The law mathematically expresses this relationship helping to understand and predict the behavior of electrolyte solutions. Imagine a crowd where each person represents an ion. The total crowd noise (conductivity) is the sum of the individual noises (molar conductivity) of each person (ion). Kohlrausch’s Law provides a framework to analyze and interpret the conductive property of the solutions, aiding in the study of electrolytes in chemistry and electrochemistry. The law is typically expressed as:
∧m = ∑zi∧i
Here, ∧m is the molar conductivity of the electrolyte, ∑ signifies the sum over all ions, zi represents the charge of each ion, and ∧i is the molar conductivity of the individual ion.
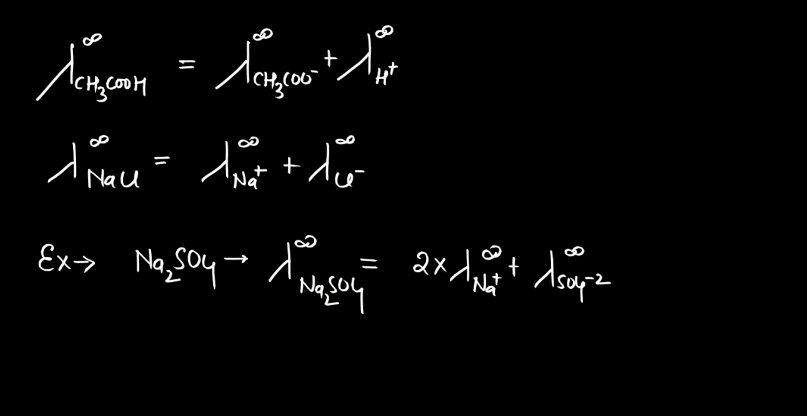
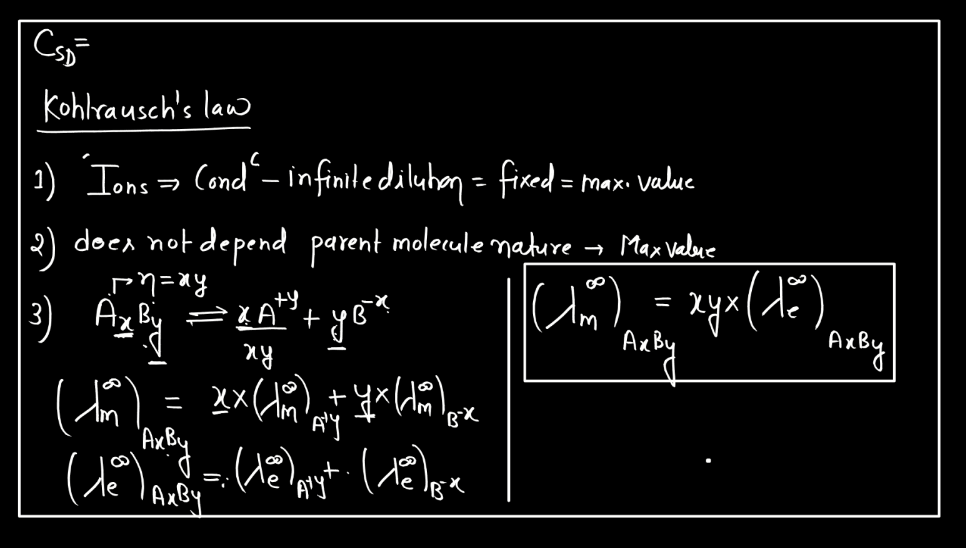
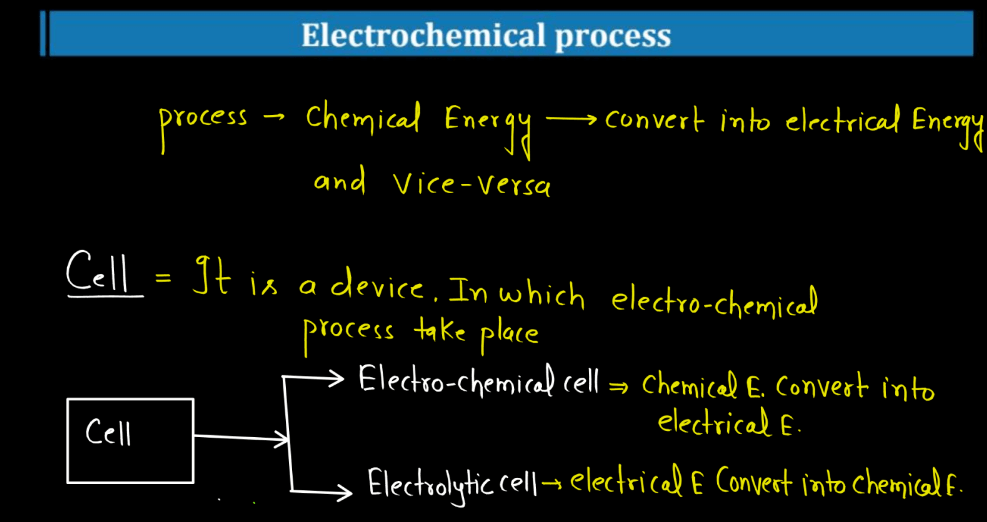
Cell
An electrochemical cell in chemistry is a device converting chemical energy to electricity. Comprising of electrodes namely anode and cathode in an electrolyte solution, it facilitates redox reactions. In galvanic cells, spontaneous reactions generate electrical energy, while electrolytic cells use external energy for non-spontaneous reactions. This technology powers batteries and fuel cells, finding wide applications. The anode undergoes oxidation, releasing electrons, and the cathode undergoes reduction, accepting electrons, and creating an electric current. Understanding cell mechanisms is vital for advancements in energy storage, electronics, and various industries reliant on efficient energy conservation.
Galvanic Cell
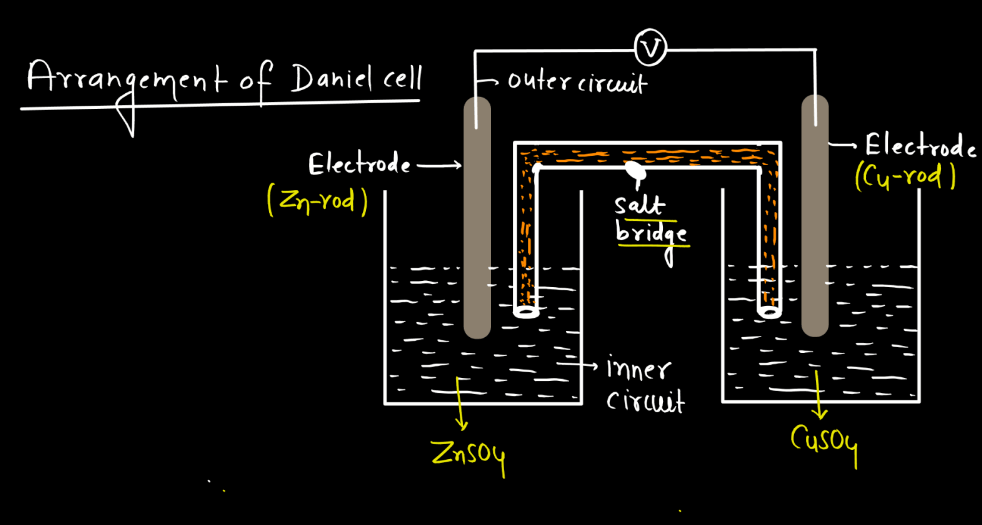
A galvanic cell is a device that turns chemical energy into electrical energy. It consists of two half-cells, each consists of two half-cells, each containing a metal electrode and a solution with ions. These half-cells are connected by a salt bridge or porous barrier. One half-cell undergoes oxidation, losing electrons and creating positive ions. The other half-cell undergoes reduction, gaining these electrons and forming negative ions. The flow of electrons between the half-cells through an external wire generates an electric current.
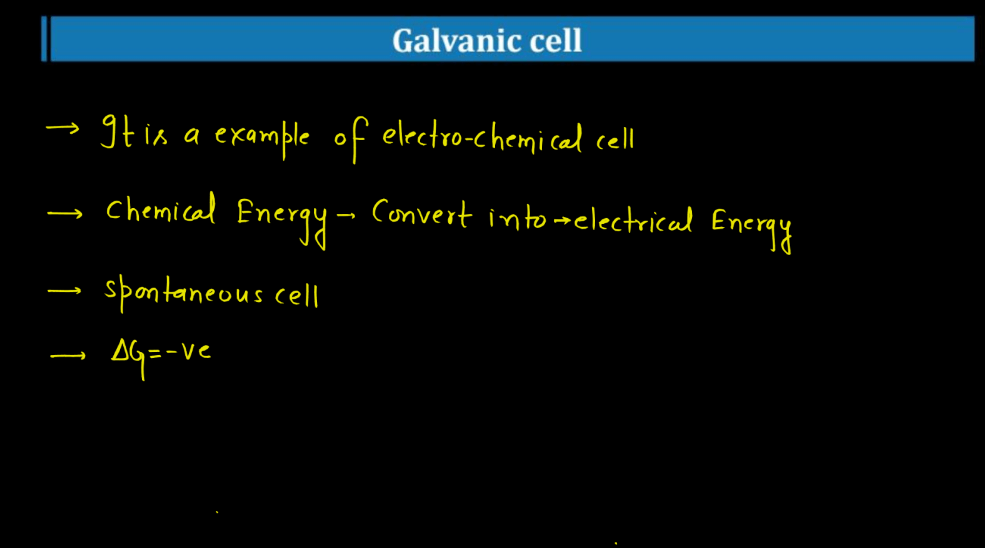
In simple terms, imagine it as a tiny power plant where metals react with solutions to produce electricity. It’s like a chemical dance where one substance loses electrons, and another gains them. The salt bridge helps maintain balance by allowing ions to move between the half-cells, ensuring a continuous flow of electrons. Overall, galvanic cells are the fundamental components of batteries, harnessing chemical reactions to power various devices in our everyday lives. There are various types of galvanic cells, each designed for specific applications. Some of the types of galvanic cells are Denial cells, Laclanche cells, Alkaline cells, Fuel Cells, etc.
Electrode [Half Cell]
An electrode in a half-cell is like a tiny energy hub in a battery. Imagine it as a small, specialized conductor with a crucial role in creating electrical power. In simple terms, it’s the part of a battery where chemical reactions happen, generating electrons that flow to produce electricity. In more detail, a half-cell consists of an electrode and its surrounding environment. One type of electrode, the anode, is where oxidation occurs.
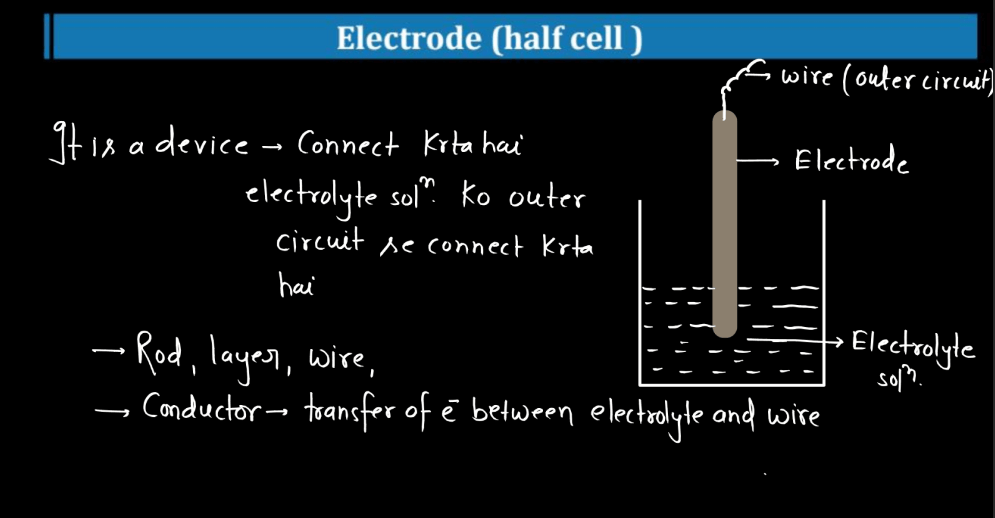
Here, atoms lose electrons, and these electrons travel through a wire, creating an electric current. The other electrode, the cathode, experiences reduction, gaining the electrons released by the anode. Together, these reactions make a battery work. So, picture electrodes as the dynamic players in a battery’s chemistry game, enabling the flow of electrons and powering our devices by converting chemical energy into electrical energy.
| Electrodes | |
| Electrodes | Description |
| Inert Electrodes | Materials like platinum or gold often serve as inert electrodes, remaining unchanged during electrochemical reactions, providing stability and conductivity. |
| Normal Electrodes | Normal electrodes are basic conductors used in everyday applications. They facilitate the flow of electricity in various devices, from simple circuits to household items, by connecting to power sources. |
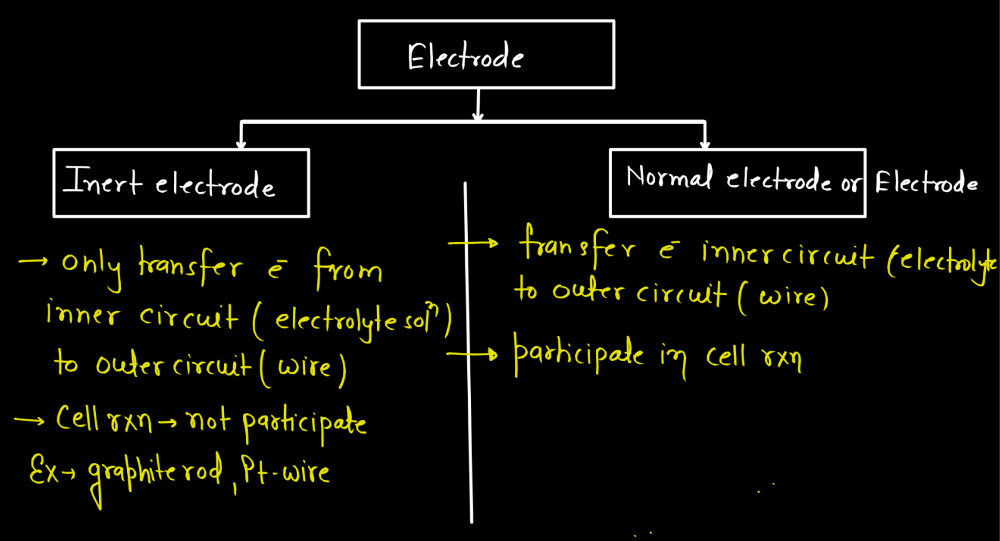
Types of Electrodes
Electrodes come in various types each serving specific purposes in different applications. These electrode types play diverse roles in batteries, sensors, electroplating, and various electrochemical processes.
| Different Types of Electrodes | |
| Types | Description |
| Metal-Metal Ion Electrode | A metal-metal ion electrode is a half-cell in electrochemistry where a metal is in contact with its own ions. It’s employed to study redox reactions and measure ion concentrations. |
| Gas-Gas Ion Electrode | A gas-gas ion electrode is a half-sell in electrochemistry involving a gas reacting with its ions. It’s used to measure gas concentrations and study redox reactions in gaseous environments. |
| Reduction Oxidation Electrode | A reduction-oxidation (redox) electrode is a half-cell in electrochemistry where reduction and oxidation reactions occur. It helps measure electron transfer in chemical reactions, often used in batteries and sensors. |
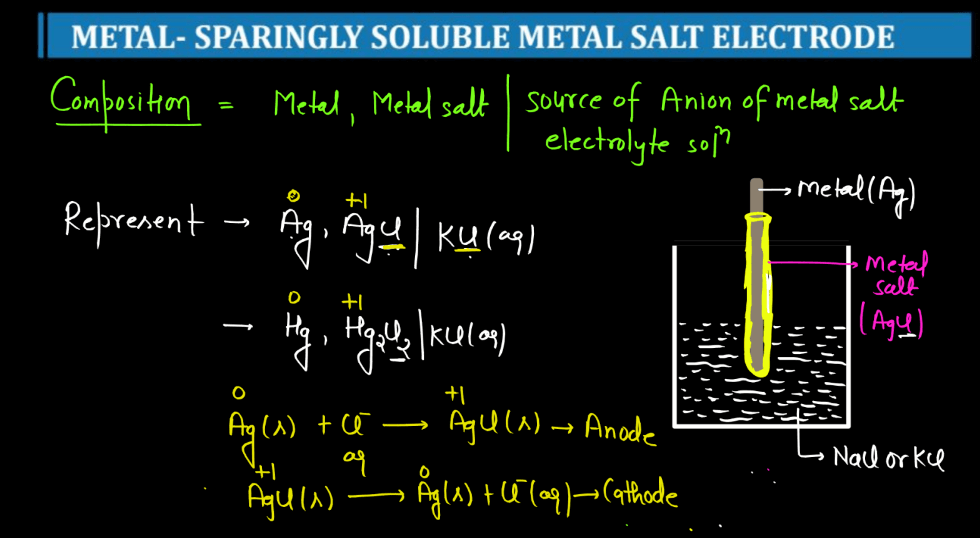
| Metal Sparingly Soluble Metal Salt Electrode | A sparingly soluble metal salt electrode is a half-cell in electrochemistry that involves a metal with a low solubility in its salt. It’s used to study reactions with limited ion dissolution. |
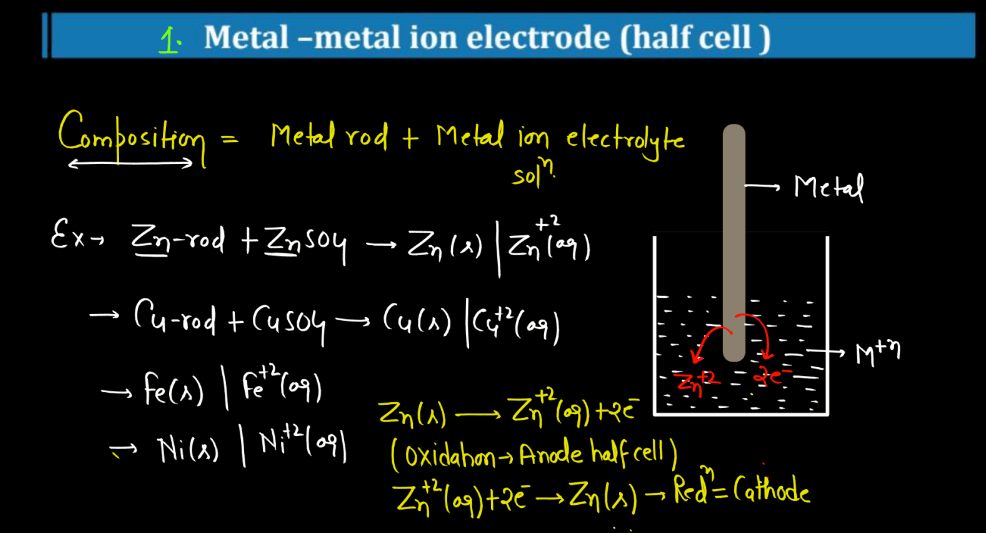
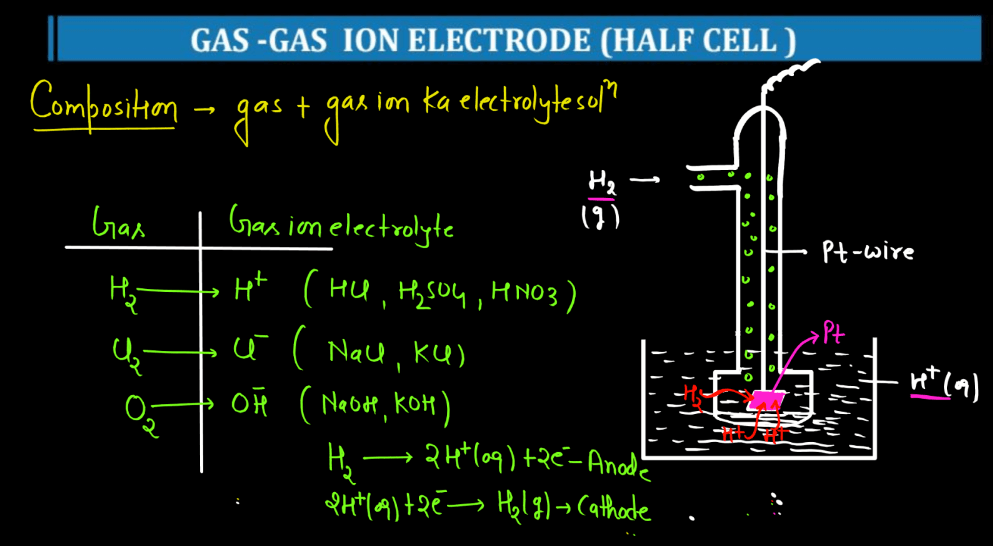
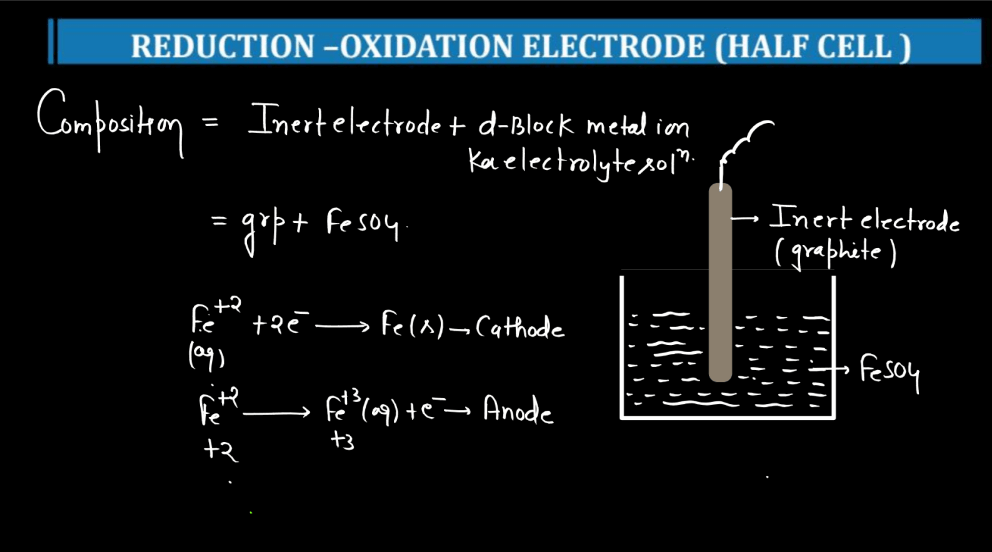
For more details about Electrochemistry Class 12, Watch the video below:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













