What is Effective Nuclear Charge
Effective Nuclear charge refers to the net positive charge experienced by an electron in an atom. It takes into account both the attractive force of the positively charged nucleus and the repulsive forces from other electrons. As electrons occupy different energy levels, inner electrons shield outer electrons from the full positive charge of the nucleus. Therefore, the effective nuclear charge felt by an outer electron is less than the actual nuclear charge. This concept helps explain trends in atomic properties, such as ionization energy and atomic size, in the periodic table. In simple terms, it is the “felt” positive charge that influences an electron’s behavior within an atom.
Slater’s Rule
Slater’s rule, formulated by John C. Slater, is a method used in quantum chemistry to estimate the effective nuclear charge experienced by an electron in a multi-electron atom. It takes into account the shielding effect caused by inner-shell electrons, influencing the attraction between the outer electron and the Nucleus. The rule assigns screening constants to different electron shells, with inner electrons shielding the outer ones from the full nuclear charge. By summing these screening constants, slater’s rule provides an approximation of the effective nuclear charge for an electron in a particular orbital. While it has limitations and simplifications, Slater’s rule serves as a practical tool for predicting atomic properties and understanding electron behavior within atoms.
How to Calculate Effective Nuclear Charge
The effective nuclear charge (Zeff) is the net positive charge experienced by an electron in an atom. To calculate the effective nuclear charge, follow the steps mentioned below. This calculation gives you the effective nuclear charge experienced by an electron in a particular energy level of the atom.
Step 1: Identify the Atomic Number: This is the number of protons in the nucleus, which is also the atomic number of the element.
Step 2: Consider Electron Shielding: Electrons in inner energy levels partially shield outer electrons from the full nuclear charge. For each inner shell, substrate one from the total shielding constant. For example, the 1s orbital has no shielding of 1, 3s, 3p, and 3d has a shielding of 2, and so on.
Step 3: Calculate Effective Nuclear Charge (Zeff): Substrate the shielding constant from the atomic number (Z).
(Zeff) = Z – S
Effective Atomic Number
The effective atomic number is like a superhero name for an atom. Imagine an atom as a bustling city with different energy levels, and electrons are its citizens. Now, not all citizens have the same power or influence. The effective atomic number is a way to show how much power an electron really holds, considering its energy level and surroundings. It’s like giving each electron a ranking based on its neighborhood and energy address. This helps scientists understand how likely an electron is to interact with others. So, when we talk about the effective atomic number, we’re basically figuring out the electron’s true strength in the atomic community, making it easier to predict its behavior.
Formula For Effective Atomic Number
Z−O.N+2 C.N
Z = Atomic number
O.N= Oxidation number
C.N = Coordination number
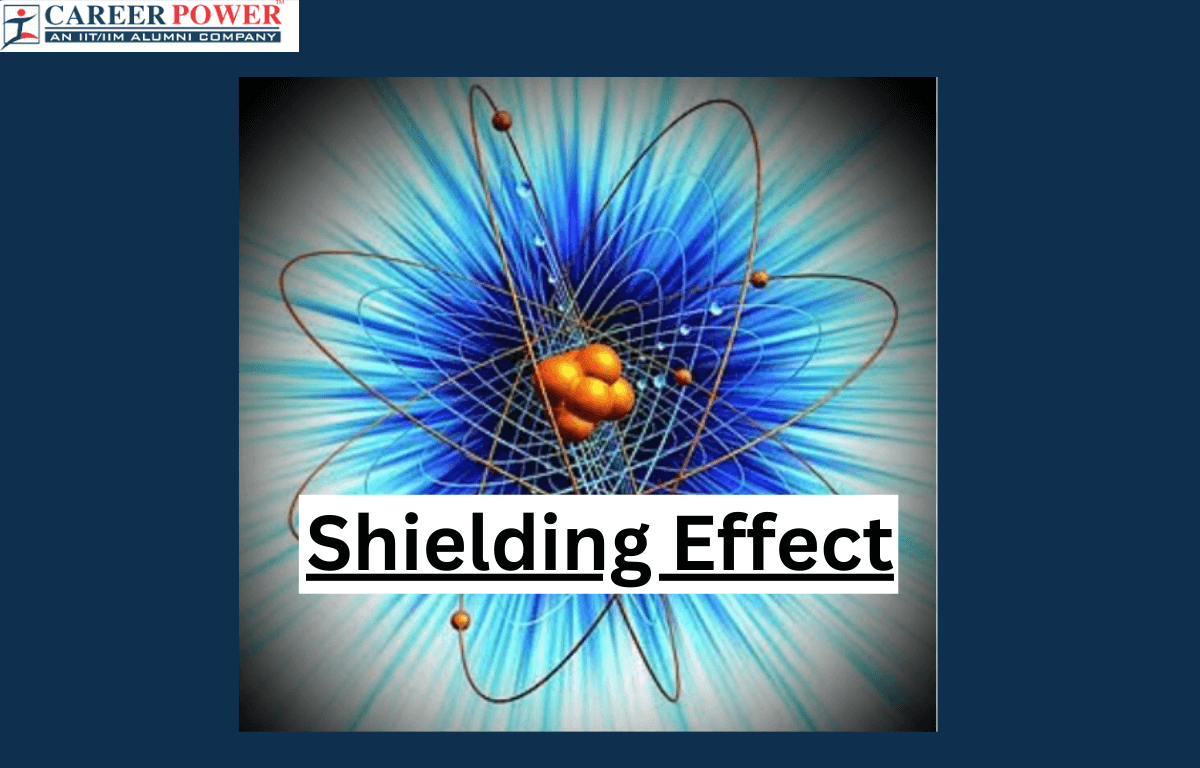
Define Shielding Effect
The shielding effect refers to how electrons in an atom shield or block the attraction between the positively charged nucleus and outer electrons. Imagine the nucleus as a magnet, and the electrons as tiny magnets around it. The inner electrons act like a barrier, reducing the pull between the nucleus and outer electrons. It’s like having layers of bodyguards – the inner electrons are the closet guards, and their presence weakens the influence of the nucleus on the outer electrons. This makes it easier for outer electrons to stay in their orbit. So, the more inner layers of electrons there are, the stronger the shielding effect, and the less the outer electrons feel the nucleus’s pull.
Some Interesting Facts About Shielding Effect
Understanding the shielding effect is crucial for explaining various properties and trends in the periodic table.
- The shielding effect is a result of inner electrons repelling outer electrons. Inner electrons partially shield outer electrons from the attraction of the positively charged nucleus.
- Shielding affects the effective nuclear charge experienced by an electron. The effective nuclear charge is the net positive charge experienced by an electron, which is reduced by the shielding effect.
- Shielding tends to increase down a group in the periodic table. As you move down a group, electrons occupy higher energy levels, increasing the number of inner electrons and enhancing the shielding effect.
- Elements with strong shielding typically have lower ionization energy, as the outer electrons are easier to remove due to reduced attraction to the nucleus.
- Shielding contributes to atomic size. Greater shielding leads to a larger atomic size, as the outer electrons experience weaker attraction to the nucleus, causing the electron cloud to spread out more.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













