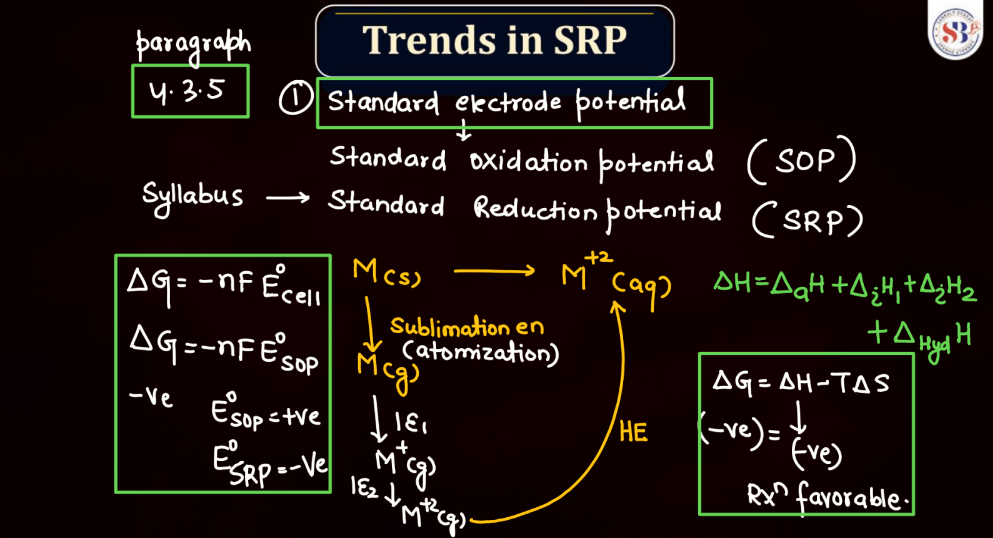
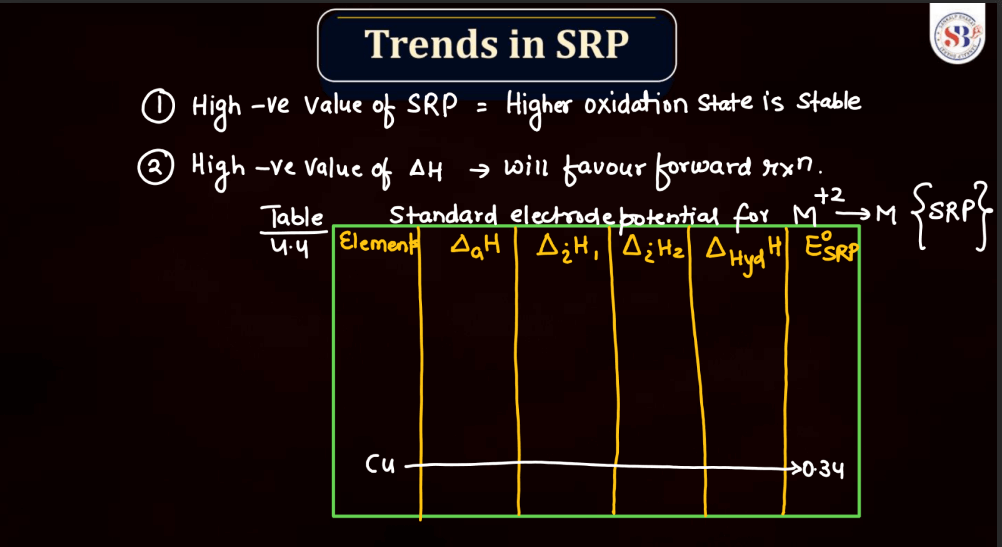
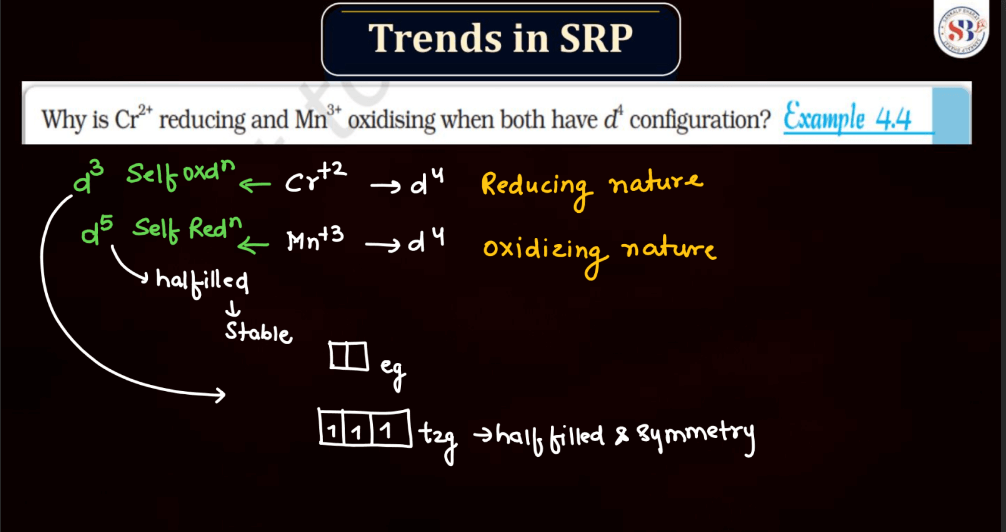
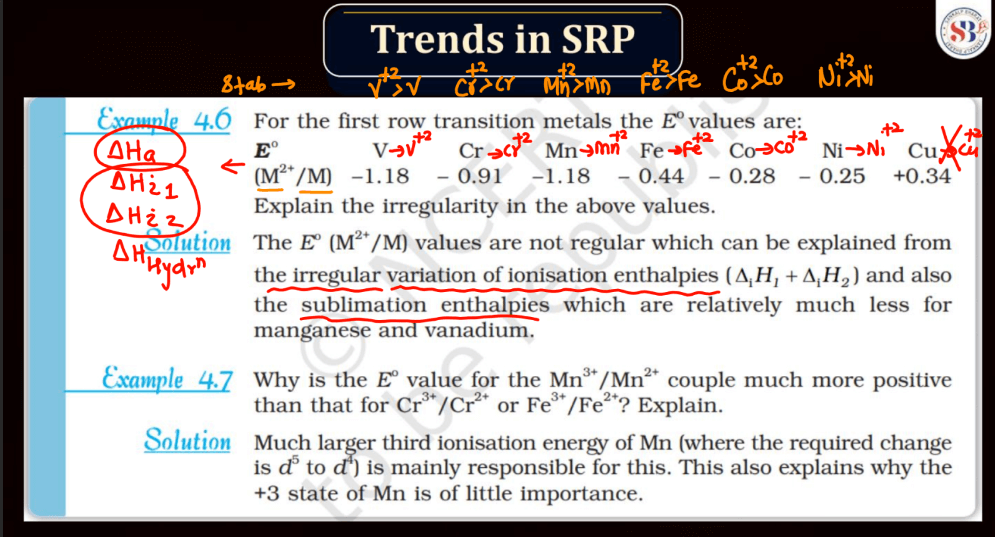
Trends of Standard Reduction Potential
In general, standard reduction potential (SRP) in the d and f block elements exhibit trends based on their electron configurations. In the d-block, SRP tends to become more positive across a period from left to right due to increasing ionization energy and decreasing atomic size. Within a group, SRP becomes more negative as you move down due to higher electron-shielding effects. In the f-block, the trends are less straightforward due to the complexity of electron configurations in the lanthanides and actinides. However, similar principles of increasing ionization energy and decreasing atomic size across a period and increasing electron shielding down a group can influence SRP trends.
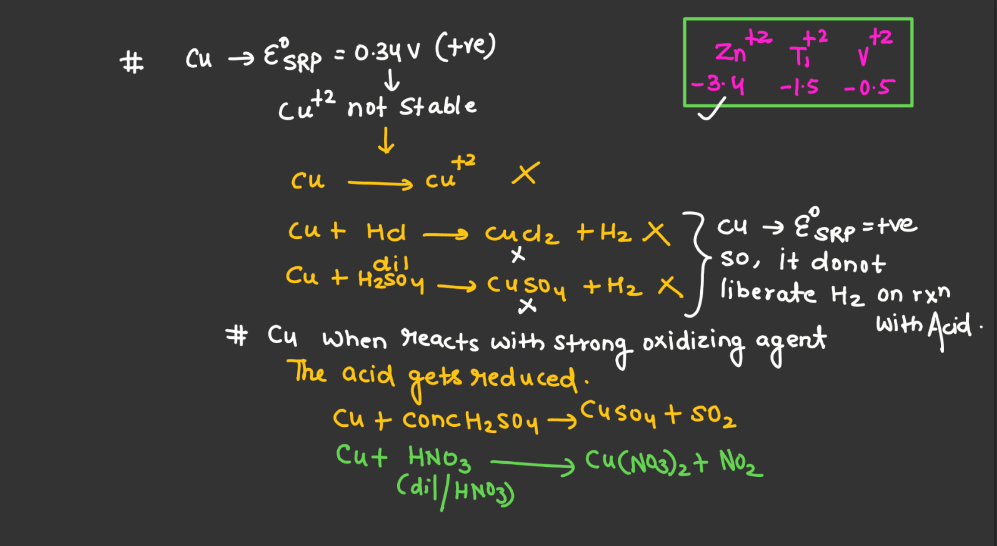
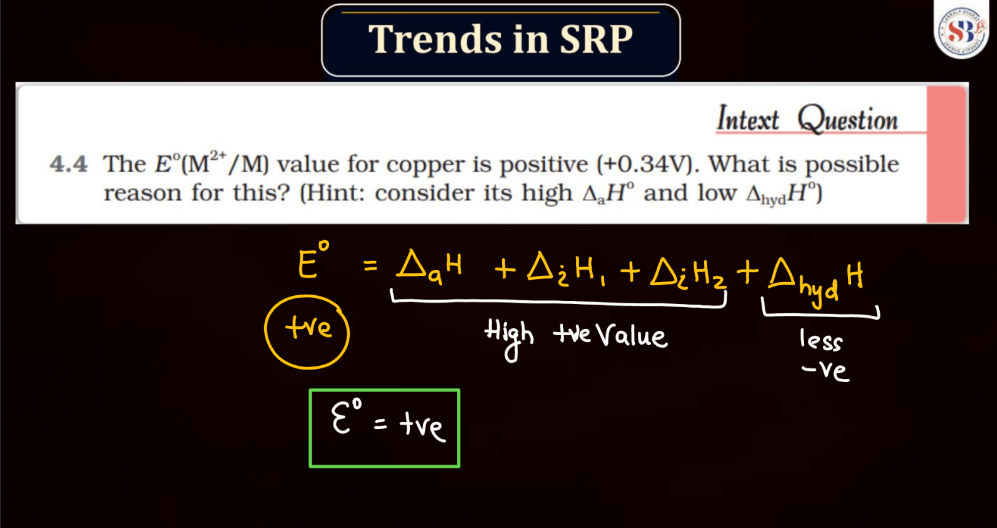
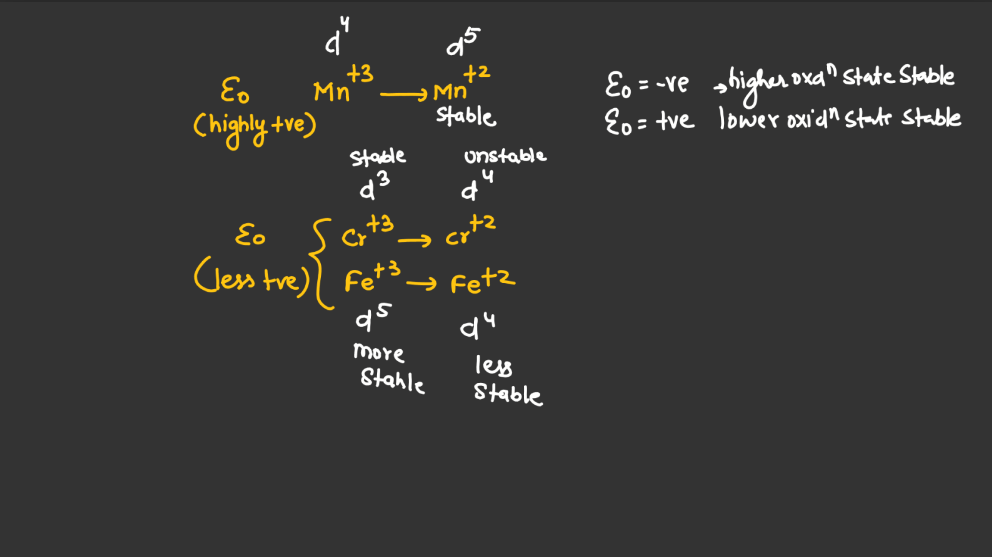

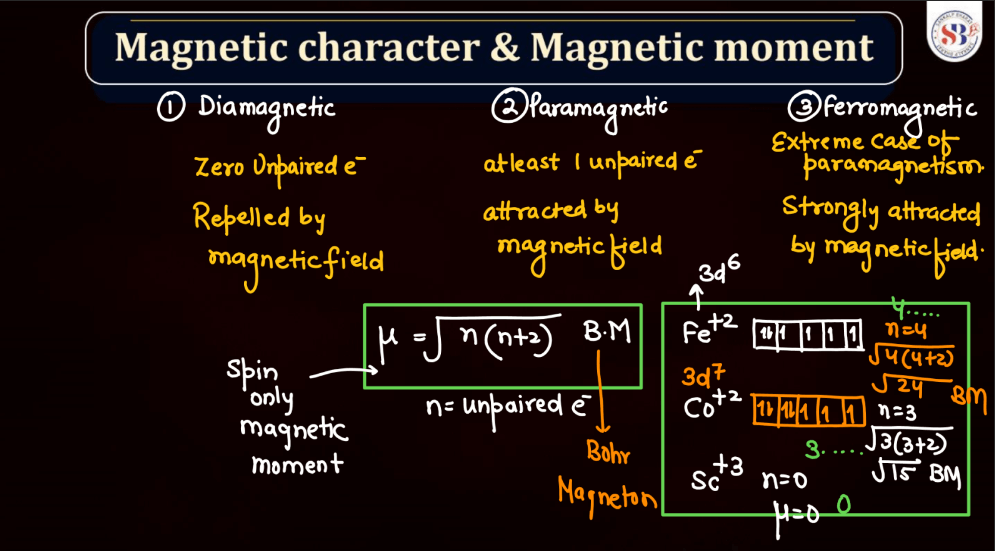
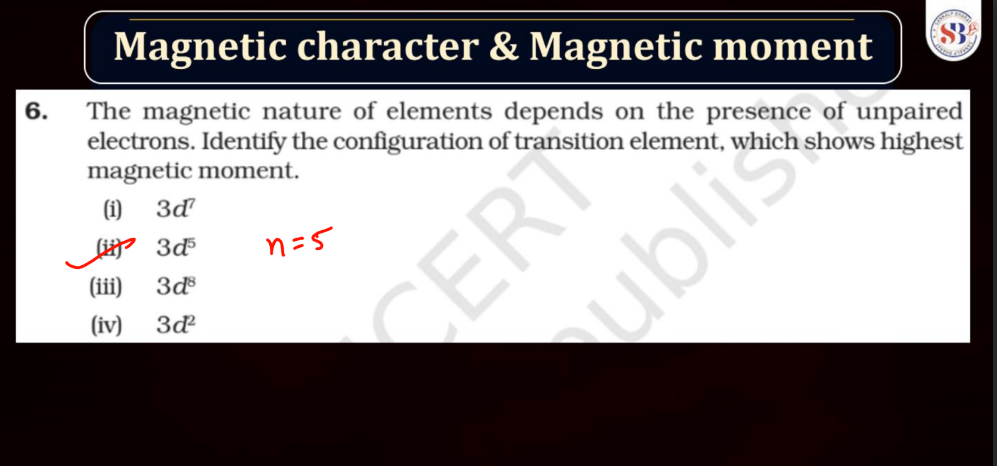
Magnetic Character and Magnetic Moment
Magnetic character refers to the ability of a substance to exhibit magnetic properties. Materials can be classified into three main categories based on their magnetic behavior: Paramagnetic, Diamagnetic, and Ferromagnetic. Ferromagnetic materials, like iron, are strongly attracted to magnets, while paramagnetic materials are weakly attracted. Diamagnetic materials, such as copper, are repelled by magnets.
Magnetic Moment, on the other hand, represents the strength and orientation of a magnetic dipole within an atom or a molecule. It arises from the motion of charged particles, like electrons, within these entities. The magnetic moment influences how a material responds to an external magnetic field. In simple terms, it’s like a tiny bar magnet associated with each atom, contributing to the overall magnetic behavior of the substance.
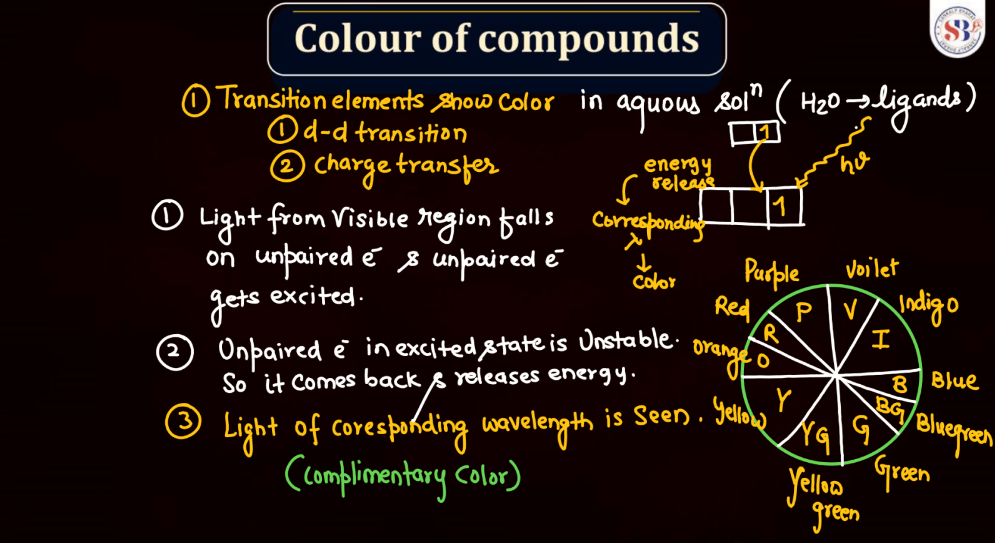
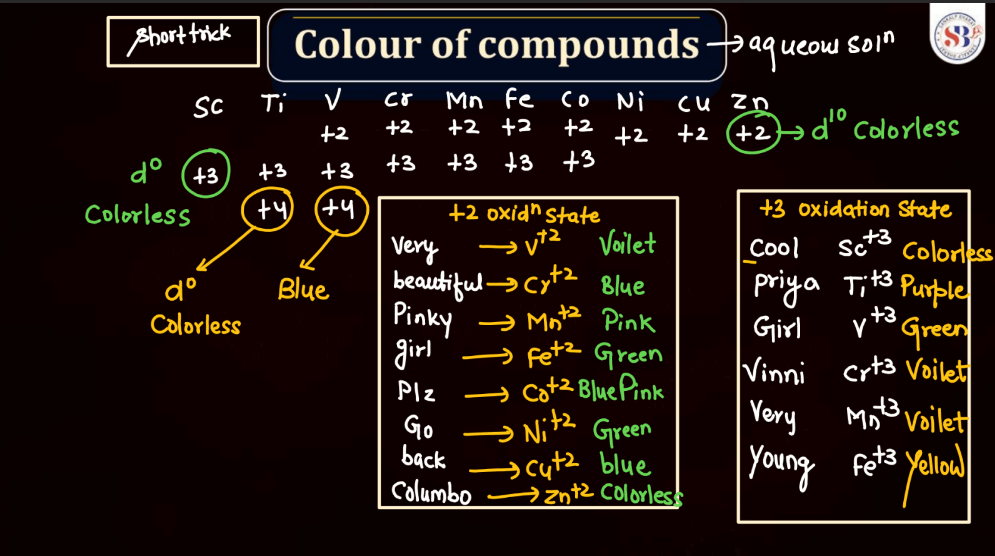
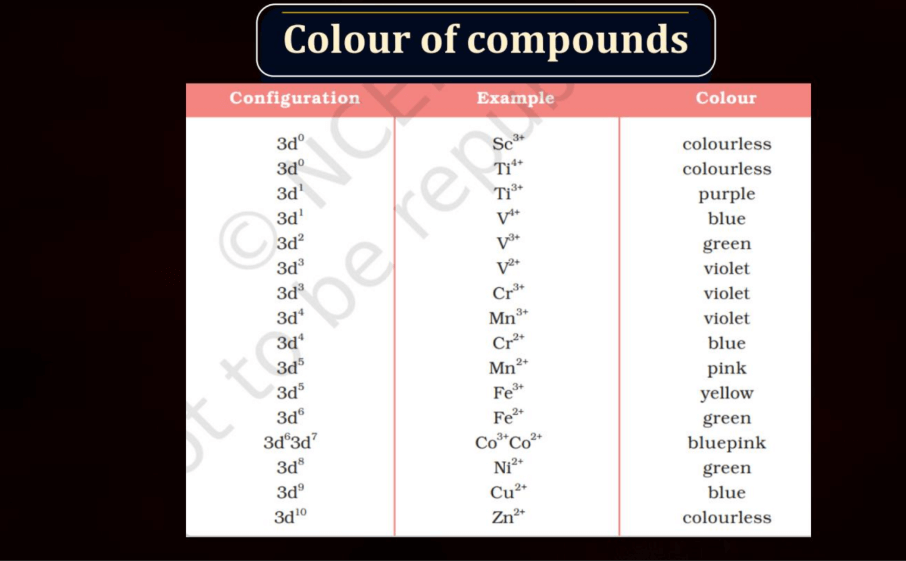
Color of Compounds
The color of compounds in d and f block elements is often attributed to electronic transitions involving d or f orbitals. Transitions metal compounds, found in the d-block, exhibit vibrant colors due to transitions between different d orbitals as electrons absorb and emit light. The f-block elements, particularly lanthanides, and actinides, also contribute to colorful compounds, often displaying characteristic dues. These colors result from complex electronic configurations and transitions within the f orbitals. Overall, the variety of colors observed in d and f block element compounds adds an intriguing dimension to the study of transition metal and rare earth element chemistry.



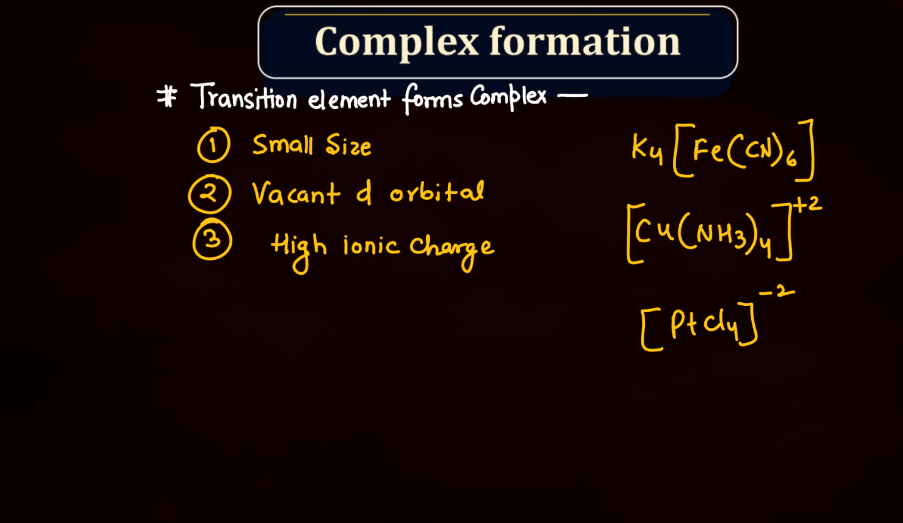
Complex Formation
The complex formation of d and f block elements involves the coordination of these transition metals with ligands to form coordination compounds. In these elements, the outermost d or f orbitals play a crucial role in bonding. Transition metals can form coordination complexes due to their ability to accept electron pairs from surrounding ligands, which are typically molecules or ions with lone pairs of electrons. The d and f orbitals allow these metals to expand their coordination number, forming stable stable complexes with various geometries. The resulting coordination compounds exhibit unique properties, such as color and magnetic behavior, making them essential in numerous chemical and biological processes.
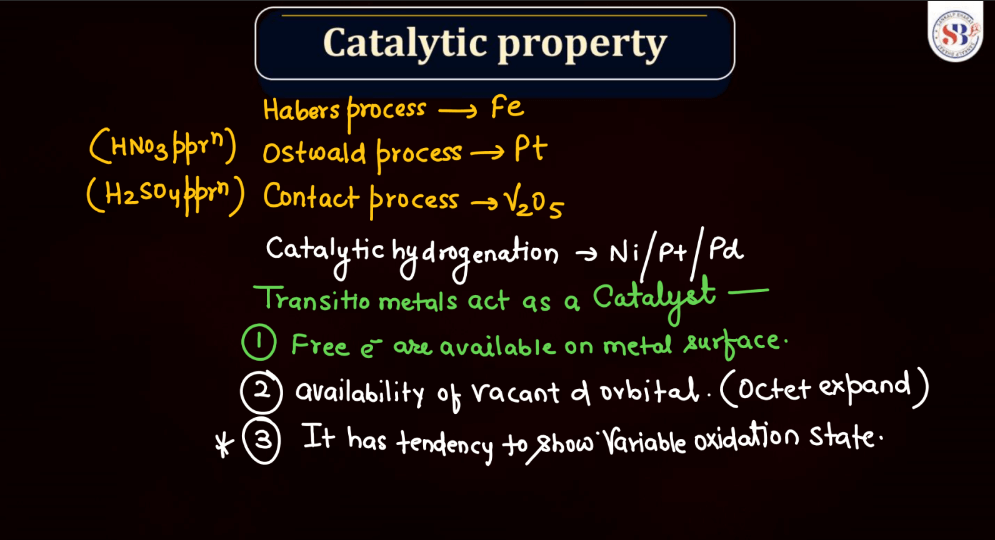
Catalytic Property
Both d and f block elements are renowned for their catalytic properties, playing crucial roles in various chemical reactions. Transition metals in these blocks often act as catalysts by providing a surface for reactions to occur, facilitating the breaking and forming of bonds. Their ability to undergo changes in oxidation states makes them versatile catalysts in redox reactions. Additionally, the d and f orbitals enable these elements to form stable intermediates during catalysis. Homogeneous and heterogeneous catalysis involving transition metals in these blocks are prevalent, influencing industrial processes and environmental remediation efforts. The catalytic activity of d and f block elements contributes significantly to advancements in chemical synthesis, energy production, and pollution control.
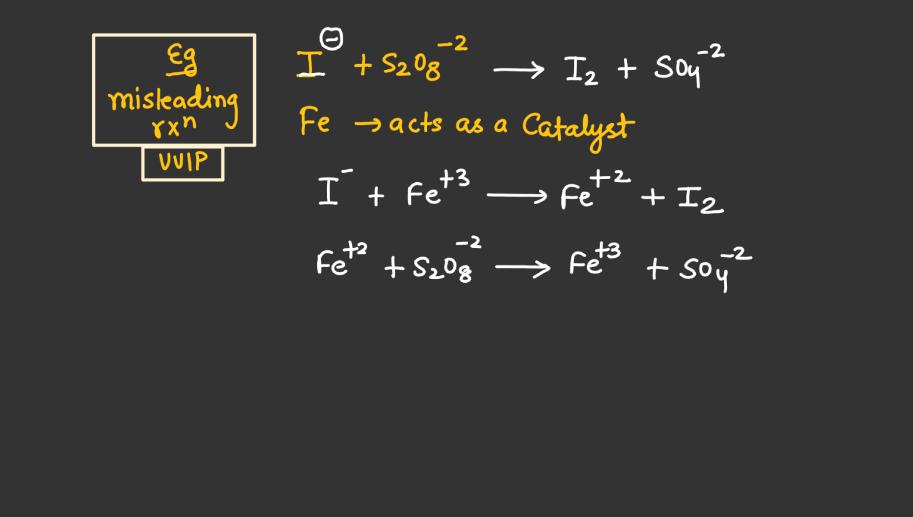
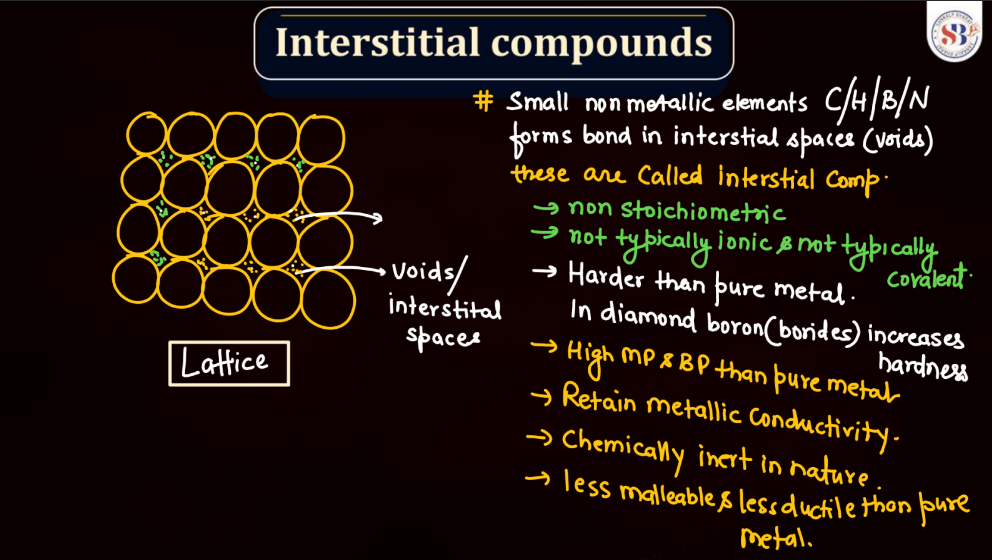
Interstitial Compounds
Interstitial compounds are formed when small atoms or molecules occupy the interstitial spaces (gaps) within the crystal lattice of a host material, usually a metal. In the context of d and f block elements, metals like transition metals often form interstitial compounds. These small atoms, such as hydrogen, boron, or carbon, fit into the gaps between the larger metal atoms without significantly disrupting the crystal structure. This can lead to changes in the physical and mechanical properties of the host material, impacting factors like hardness, electrical conductivity, and magnetic properties. Interstitial compounds are crucial in materials science and play a crucial role in the development of new materials with tailored properties for specific applications.

Alloy Formation
The alloy formation involves mixing d and f block elements to create materials with enhanced properties. In alloys, different metals, often from the transition metal groups, combine to form a solid solution. This blending alters the properties of individual elements, resulting in improved strength, durability, and other desired characteristics. For instance, steel, an alloy of iron with small amounts of carbon and other elements, exhibits enhanced strength and corrosion resistance compared to pure iron. The versatility of d and f block elements in alloy formation allows engineers and scientists to tailor materials for specific applications, from aerospace components to everyday objects. This ability to fine-tune properties makes alloying a vital aspect of material science and technological advancements.

To know more, watch the complete video about the Inorganic Chemistry:


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













