CBSE Class 10 Science Answer Key 2024: The Central Board of Secondary Education has successfully conducted the CBSE Class 10 Board Science examination 2024 today i.e., March 02, 2024. The CBSE Class 10th Science exam was conducted from 10:30 am to 01:30 pm at different exam centers. As the CBSE Class 10th Science Board exam has been successfully conducted all the CBSE Class 10th students can download the complete CBSE Class 10 Science Answer Key 2024 with Complete Question Paper Analysis for All SETs 1, 2, and 3 from the direct link that has been shared in the article below.
CBSE Class 10 Science Answer Key 2024
The CBSE Class 10th Science Board Examination 2024 has been successfully conducted today i.e., March 02, 2024 at 01:30 pm. The CBSE Board Class 10th science board exam 2024 was scheduled to be conducted in offline mode i.e., pen and paper mode. Students were allotted a time duration of 3 hours to complete the exam whereas an extra 15 minutes were given for reading the question paper. As per CBSE, the Class 10th science syllabus comprised a total of 13 chapters categorized into Physics, Chemistry, and Biology. The CBSE Class 10th Science Board question paper included both objective and subjective types of questions. Students will get the direct link to download the PDF of CBSE Class 10the Science Question Paper 2024 along with the answer key here.
Class 10 Science Answer Key 2024
The CBSE Class 10th Science question paper consists of a total of 39 questions that were further divided into 05 different sections. Where Section A consists of 20 objective-type questions carrying 1 mark each, Section B comprises 06 Very Short questions carrying 02 marks each. The CBSE Board Class 10 Science Question Paper 2024 followed the same pattern as given in the CBSE Class 10th Science Board Model Paper 2024.
| CBSE Class 10 Science Answer Key 2024 | |
| Exam Conducting Body | Central Board of Secondary Education |
| Name of the Examination | CBSE Class 10th Board Examination 2024 |
| Category | Answer Key |
| Status | Released |
| CBSE Class 10th Science Exam Date 2024 | March 02, 2024 |
| CBSE Class 10th Science Answer Key release date 2024 | March 2024 |
| Mode of Answer Key Availability | Online Mode |
| Official Website | https://www.cbse.nic.in/ |
Class 10 Science Answer Key 2024 All SETs 1,2,3
Our subject expert’s faculty has prepared the specially designed CBSE Class 10 Science Answer Key 2024 for the questions that are asked in the CBSE Class 10th Science Exam. The CBSE Class 10 Science Answer Key 2024 PDF can easily be downloaded from the direct link that has been shared here.
CBSE Class 10 Science Answer Key 2024 SET 31/5/1
SECTION A
Select and write the most appropriate option out of the four options given for each of the questions no. 1 to 20.
1. To balance the following chemical equation, the values of the coefficients x, y and z must be respectively:
x Zn(NO3)2 → y ZnO + NO2 + O2
(A) 4, 2, 2
(B) 4, 4, 2
(C) 2, 2, 4
(D) 2, 4, 2
Answer: (C) 2, 2, 4
2. Which of the following is a redox reaction, but not a combination reaction?
(A) C+ O2→CO2
(B) 2 Mg + O2 → 2 MgO
(C) 2H2 + O2→2 H2O
(D) Fe2O3+ 3CO → 2 Fe + 3CO2
Answer: (D) Fe2O3+ 3CO → 2 Fe + 3CO2
3. The salt present in tooth enamel is:
(A) Calcium phosphate
(B) Magnesium phosphate
(C) Sodium phosphate
(D) Aluminium phosphate
Answer: (A) Calcium phosphate
4. An aqueous solution of sodium chloride is prepared in distilled water. The pH of this solution is:
(A) 6
(B) 8
(C) 7
(D) 3
Answer: (C) 7
5. A metal ‘X’ is used in thermit process. When ‘X’ is heated with oxygen, it gives an oxide ‘Y’, which is amphoteric in nature. ‘X’ and ‘Y’ respectively are:
(A) Mn, MnO2
(B) Al, Al2O3
(C) Fe, Fe2O3
(D) Mg, MgO
Answer: (B) Al, Al2O3
6. The process in which transport of soluble products of photosynthesis takes place in plants is known as:
(A) Transpiration
(B) Evaporation
(C) Conduction
(D) Translocation
Answer: (D) Translocation
7. The correct sequence of events when someone’s hand touches a hot object unconsciously:
(A) Receptors in skin Motor neuron → Relay neuron → Sensory neuron Effector muscle in arm
(B) Receptors in skin Relay neuron Sensory neuron → Motor neuron Effector muscle in arm
(C) Receptors in skin Sensory neuron Relay neuron → Motor neuron Effector muscle in arm
(D) Receptors in skin Sensory neuron → Effector muscle in arm → Motor neuron → Relay neuron
Answer: (C) Receptors in skin → Sensory neuron → Relay neuron → Motor neuron Effector muscle in arm
8. The sense organ in which olfactory receptors are present is:
(A) Nose
(B) Skin
(C) Tongue
(D) Inner ear
Answer: (A) Nose
9. The incorrect statement about placenta is:
(A) It is a disc embedded in the uterine wall.
(B) It contains villi on the embryo’s side of the tissue.
(C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
(D) The embryo gets nutrition from the mother’s blood through it.
Answer: (C) It has a very small surface area for glucose and oxygen to from mother to the embryo.
10. Select from the following the conditions responsible for the rapid spread of bread mould on a slice of bread:
(i) Formation of large number of spores
(ii) Presence of moisture and nutrients in bread
(iii) Low temperature
( iv) Presence of hyphae
(A) (i) and (ii)
(B) (ii) and (iv)
(C) (ii) and (iii)
(D) (iii) and (iv)
Answer: (A) (i) and (ii)
11. How will the image formed by a convex lens be affected, if the upper half of the lens is wrapped with a black paper?
(A) The size of the image formed will be one-half of the size of the image due to complete lens.
(B) The image of upper half of the object will not be formed.
(C) The brightness of the image will reduce.
(D) The lower half of the inverted image will not be formed
Answer: (C) The brightness of the image will reduce.
For Questions number 17 to 20, two statements are given one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A): Some vegetable oils are healthy.
Reason (R): Vegetable oils generally have long unsaturated carbon chains.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
18. Assertion (A): Sex of the children will be determined by what they inherit from their mother.
Reason (R): Women have XX sex chromosomes.
Answer: (D) Assertion (A) is false, but Reason (R) is true.
19. Assertion (A): Electrons move from lower potential to higher potential in a conductor.
Reason (R): A dry cell maintains electric potential difference across the ends of a conductor.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
20. Assertion (A): Ozone layer protects the surface of the Earth from harmful UV radiations.
Reason (R): Chlorofluorocarbons (CFCs) are responsible for depletion of ozone layer
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
SECTION B
Questions no. 21 to 26 are very short answer-type questions.
21. (a) Copper powder is taken in a china dish and heated over a burner. Name the product formed and state its colour. Write the chemical equation for the reaction involved.
OR
(b) Write chemical equation for the chemical reaction which occurs when the aqueous solutions of barium chloride and sodium sulphate react together. Write the symbols of the ions present in the compound precipitated in the reaction.
22. The melting and boiling points of carbon compounds are generally low and they are largely non-conductors of electricity. State two conclusions based on these two properties.
23. (a) Sometimes while running, the athletes suffer from muscle cramps. Why? How is the respiration in this case different from aerobic respiration?
OR
(b) Write the other name given to lymph. State its two functions.
24. Some unicellular organisms such as Plasmodium and Leishmania differ in the manner in which they reproduce. Name and explain the reproductive process taking place in them.
25. The heat produced at a point due to concentration of sunlight by a convex lens burns a paper.
(a) Explain why it happens.
(b) Name the term (in the context of the lens used) given to the point at which the paper starts burning. What does the bright spot formed on the paper represent?
26. An electric source can supply a charge of 500 coulomb. If the current drawn by a device is 25 mA, find the time in which the electric source will be discharged completely.
Section – C
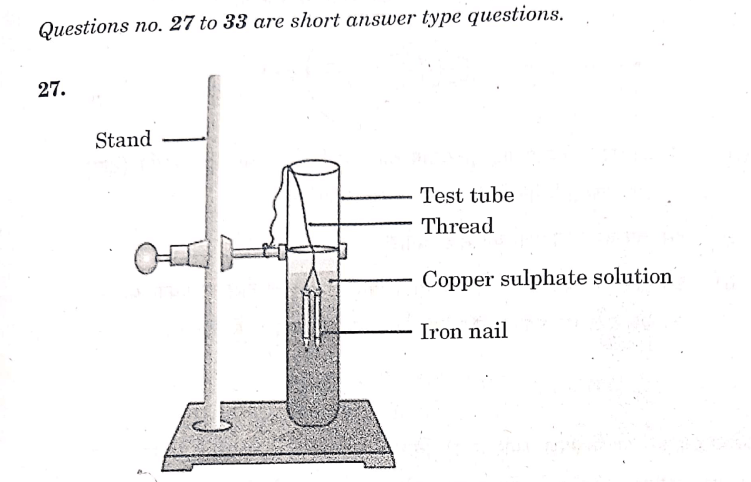
Study the experimental set-up shown in the diagram and write chemical equation for the chemical reaction involved. Name and define the type of reaction. List two other metals which can be used in place of iron to show the same type of reaction with copper sulphate solution.
28. Name the ore of mercury and state the form in which it is found in nature. Write the chemical equations along with the condition required for the reactions involved in the extraction of mercury from its ore.
29. Taking the example of any two animal hormones along with their gland of secretion, explain how these hormones help
(i) in growth and development and
(ii) regulate metabolism, in the body.
30. Mendel crossed pure tall pea plants (TT) with pure short pea plants (tt) and obtained F₁ progeny. When the plants of F₁ progeny were self-pollinated, plants of F₂ progeny were obtained.
(a) What did the plants of F₁ progeny look like? Give their gene combination.
(b) Why could the gene for shortness not be expressed in plants of F₁ progeny?
(c) Write the ratio of the plants obtained in F₂ progeny and state the conclusion that can be drawn from this experiment.
31. (a) Study the diagram given below and answer the questions that follow :
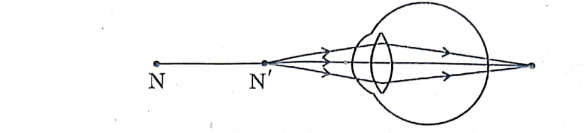
(i) Name the defect of vision depicted in this diagram stating the part of the eye responsible for this condition.
(ii) List two causes of this defect.
(iii) Name the type of lens used to correct this defect and state its role in this case.
OR
(b) What is dispersion of white light? State its cause. Draw a diagram to show dispersion of a beam of white light by a glass prism.
32. (a) What happens when a bundle of wires of soft iron is placed inside the coil of a solenoid carrying a steady current ? Name the device obtained. Why is it called so?
(b) Draw the magnetic field lines inside a current carrying solenoid. What does this pattern of magnetic field lines indicate?
33. Differentiate between food chain and food web. In a food chain consisting of deer, grass and tiger, if the population of deer decreases, what will happen to the population of organisms belonging to the first and third trophic levels?
SECTION D
Questions no. 34 to 36 are long answer type questions.
34. (a) A few crystals of ferrous sulphate were taken in a dry boiling tube and heated. Tiny water droplets were observed in the tube after some time.
(i) From where did these water droplets appear? Explain.
(ii) What colour change will be observed during heating?
(iii) How many molecules of water are attached per molecule of FeSO4 crystal? Write the molecular formula of crystalline forms of (I) Copper sulphate, and (II) Sodium carbonate.
(iv) State how is Plaster of Paris obtained from gypsum. Write two uses of Plaster of Paris.
OR
(b) An acid ‘X’ present in tamarind when mixed with ‘Y’, produces a mixture ‘Z’. ‘Z’ on addition to a dough when heated makes cakes soft and spongy. ‘Y’ is prepared from common salt and helps in faster cooking.
(i) Write the common names of ‘X’, ‘Y’ and ‘Z’, and the chemical formula of ‘Y’.
(ii) How is ‘Y’ prepared and how does it help in making cakes soft and spongy? Illustrate the reaction with suitable chemical equation.
(iii) Write the name and chemical formula of a mild base other than ‘Y’ used as an antacid.
35. (a) Design an experiment to demonstrate that carbon dioxide is essential for photosynthesis. Write the observation and conclusion of the experiment.
OR
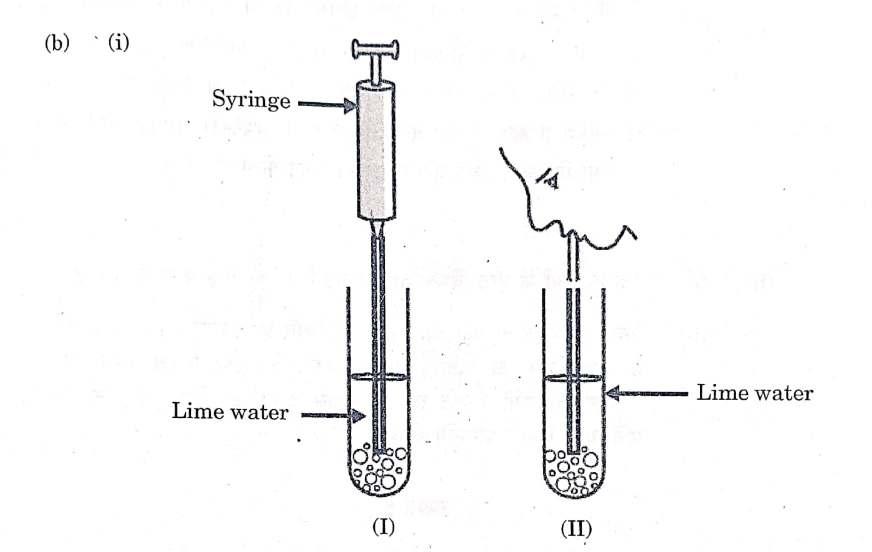
In the experimental set-up shown above in diagram (I) atmospheric air is being passed into lime water with a syringe while in diagram (II) air is being exhaled into lime water. The time taken for the lime water to turn milky in both the test tubes is different. Give reason.
(ii) Draw the diagram of an open stomatal pore and label (I) Guard cells, and (II) Chloroplast on it. Mention two functions performed by stomata.
36. (a ) (i) The potential difference across the two ends of a circuit component is decreased to one-third of its initial value, while its resistance remains constant. What change will be observed in the current flowing through it ? Name and state the law which helps us to answer this question.
(ii) Draw a schematic diagram of a circuit consisting of a battery of four 1.5 V cells, a 5 resistor, a 10 Ω resistor and a 15 Ω resistor and a plug key, all connected in, series. Now find (I) the electric current passing through the circuit, and (II) potential difference across the 10 Ω resistor when the plug key is closed.
OR
(b) (i) When is the potential difference between two points said to be 1 volt?
(ii) A copper wire has a diameter of 0.2 mm and resistivity of 1-6 × 10-8 2 m. What will be the length of this wire to make its resistance 14 Ω ? How much does the resistance change, if the diameter of the wire is doubled?
SECTION E
Questions no. 37 to 39 are case-based/data-based questions with 3 short sub-parts. Internal choice is provided in one of these sub-parts.
37. Carbon is a versatile element that forms the basis of all living organisms and many of the things we use. A large variety of compounds is formed because of its tetravalency. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements. Answer the following questions:
(a) What are hydrocarbons?
(b) List two properties by virtue of which carbon can form a large number of compounds.
(c) (i) Write the formula of the functional group present in (1) aldehydes, and (2) ketones. Write chemical equation for the reaction that occurs between ethanoic acid and ethanol in the presence of a catalyst.
OR
(c) (ii) What are structural isomers ? Write the structures of two isomers of butane (C4H10).
38. Pollination is an important process in sexual reproduction of plants. It is an essential process that facilitates fertilisation in plants. Pollinating agents can be wind, water, insects and birds. Several changes take place in the flower after the fertilization has taken place.
(a) Write the main difference between self-pollination and cross-pollination.
(b) Name the part of the flower which attracts insects for pollination. What happens to this part after fertilisation?
(c) (i) Define fertilisation. What is the fate of ovules and the ovary in a flower after fertilisation?
OR
(c) (ii) In a germinating seed, which parts are known as future shoot and future root? Mention the function of cotyledon.
39. A highly polished surface such as a mirror reflects most of the light falling on it. In our daily life we use two types of mirrors – plane and spherical. The reflecting surface of a spherical mirrors may be curved inwards or outwards. In concave mirrors, reflection takes place from the inner surface, while in convex mirrors reflection takes place from the outer surface.
(a) Define the principal axis of a concave mirror.
(b) A ray of light is incident on a concave mirror, parallel to its principal axis. If this ray after reflection from the mirror passes through the principal axis from a point at a distance of 10 cm from the pole of the mirror, find the radius of curvature of the mirror.
(c) (i) An object is placed at a distance of 10 cm from the pole of a convex mirror of focal length 15 cm. Find the position of the image.
OR
(c) (ii) A mirror forms a virtual, erect and diminished image of an object. Identify the type of this mirror. Draw a ray diagram to show the image formation in this case.
Class 10 Science Answer Key 2024 SET 31/4/2
Select and write the most appropriate option out of the four options given for each of the questions no. 1 to 20. There is no negative marking for incorrect response.
1. A chemical reaction in which exchange of ions occurs between the reactants, is known as:
(A) Endothermic Reaction
(B) Exothermic Reaction
(C) Double Displacement Reaction
(D) Displacement Reaction
Answer: (C) Double Displacement Reaction
2. A zygote is formed by the fusion of a male gamete and a female gamete. The number of chromosomes in the zygote of a human is:
(A) 23
(B) 44
(C) 46
(D) 92
Answer :(C) 46
3. The part of seed which is a source of food during germination of seed is :
(A) Cotyledon.
(B) Radicle
(C) Plumule
(D) Embryo
Answer: (A) Cotyledon.
4. The plants that can be raised by the method of vegetative propagation are:
(A) Sugarcane, roses, grapes
(B) Sugarcane, mustard, potato
(C) Banana, orange, mustard
(D) Papaya, mustard, potato
Answer:
5. A plant growth inhibitor hormone which causes wilting of leaves is called:
(A) Auxin
(B) Cytokinin
(C) Abscisic acid.
(D) Gibberellin
Answer: (C) Abscisic acid.
6. An aqueous solution of a salt turns blue litmus to red. The salt could be the one obtained by the reaction of:
(A) HNO3 and NaOH
(B) H₂SO₄ and KOH
(C) CH3COOH and NaOH
(D) HCl and NH OH
7. Four solutions, namely glucose, alcohol, hydrochloric acid and sulphuric acid filled in four separate beakers are connected one by one in an electric circuit with a bulb. The solutions in which the bulb will glow when current is passed are:
(A) Glucose and alcohol
(B) Alcohol and hydrochloric acid
(C) Glucose and sulphuric acid
(D) Hydrochloric acid and sulphuric acid
8. The metals which are found in both free state as well as combined state are:
(A) Gold and platinum
(B) Platinum and silver
(C) Copper and silver
(D) Gold and silver
9. The number of single and double bonds present in a molecule of benzene (C8Hg) respectively, are:
(A) 6 and 6
(B) 9 and 3
(C) 3 and 9
(D) 3 and 3
10. In human beings, when the process of digestion is completed, the (i) proteins, (ii) carbohydrates, and (iii) fats are respectively finally converted into:
(A) (i) Amino acids, (ii) glucose and (iii) fatty acids
(B) (i) Amino acids, (ii) glucose, (ii) fatty acids and glycerol
(C) (i) Glucose, (ii) fatty acids and glycerol, (iii) amino acids
(D) (i) Sugars, (ii) amino acids, (iii) fatty acids and glycerol
11. Some wastes are given below:
(i) Garden waste
(ii) Ball point pen refills
(iii) Empty medicine bottles made of glass
(iv) Peels of fruits and vegetables
(v) Old cotton shirt
The non-biodegradable wastes among these are:
(A) (i) and (ii)
(B) (ii) and (iii)
(C) (i), (iv) and (v)
(D) (i), (iii) and (iv)
12. A rectangular loop ABCD carrying a current I is situated near a straight conductor XY, such that the conductor is parallel to the side AB of the loop and is in the plane of the loop. If a steady current I is established in the conductor as shown, the conductor XY will
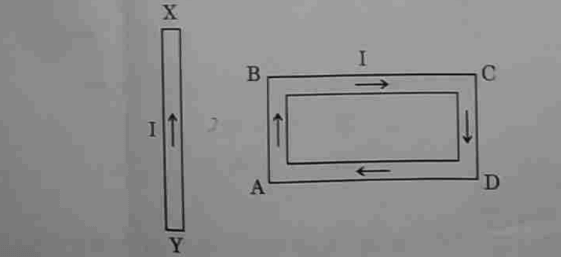
(A) remain stationary
(B) move towards the side AB of the loop.
(C) move away from the side AB of the loop.
(D) rotate about its axis.
13. Absolute refractive index of glass and water is and respectively. If 2 3 the speed of light in glass is 2 x 108m/s, the speed of light in water is:
(A) 9/4 x 108 m/s 4
(B) 5/2 × 108 m/s
(C) 7/3 x 108 m/s
(D) 16 /9 x 108 m/s
14. When a beam of white light passes through a region having very fine dust particles, the colour of light mainly scattered in that region is:
(A) Red
(B) Orange
(C) Blue.
(D) Yellow
15. Consider the following combinations of resistors:
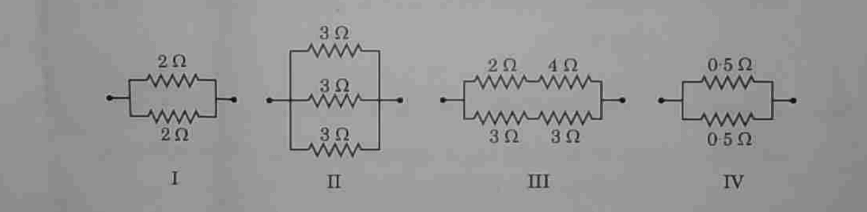
The combinations having equivalent resistance 1 2 is/are:
(A) I and IV
(C) I and II
(B) Only IV
(D) I, II and III
16. An electric iron of resistance 20 Ω draws a current of 5 A. The heat developed in the iron in 30 seconds is:
(A) 15000 J
(B) 6000 J
(C) 1500 J
(D) 3000 J
For Questions number 17 to 20, two statements are given – one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A): Oxygen is essential for all aerobic forms of life.
Reason (R): Free oxygen atoms combine with molecular oxygen to A form ozone.
18. Assertion (A): Most of the plants close their stomata at night.
Reason (R) Closing of stomata helps to conserve water as large amount of water evaporates from the leaves.
19. Assertion (A): The extraction of metals from their sulphide ores cannot take place without roasting of the ore.
Reason (R) Roasting converts sulphide ores directly into metals.
20. Assertion (A): Magnetic field lines never intersect each other.
Reason (R): If they intersect, then at the point of intersection, the compass needle would point towards two directions, which is not possible.
Class 10 Science Answer Key 2024 SET 31/2/3
Select and write one most appropriate option out of the four options given for each of the questions 1 to 20:
1. An iron nail is placed in a solution of copper sulphate. The nail is taken out after 15 minutes. The nail will be found to be covered with:
(A) blue deposit
(B) brown deposit
(C) grey deposit
(D) green deposit
2. Consider the following cases:
(a) CaSO4 + Al →
(b) CuSO,+Ca →
(c) FeSO4 + Cu →
(d) ZnSO4 + Mg →
The cases in which new products will form are –
(A) (a) and (b)
(B) (b) and (c)
(C) (c) and (d)
(D) (b) and (d)
3. Which of the following reactions is an endothermic reaction?
(A) Burning of coal
(B) Decomposition of vegetable matter into compost
(C) Process of respiration
(D) Decomposition of calcium carbonate to form quick lime and carbon dioxide.
4. The oxide which can react with HCl as well as KOH to give corresponding salt and water is
(A) CuO
(B) AlO3
(C) Na₂O
(D) K₂O
5. The juice of tamarind turns blue litmus to red. It is because of the presence of an acid called:
(A) methanoic acid
(B) acetic acid
(C) tartaric acid
(D) oxalic acid
6. Consider the following statements about homologous series of carbon compounds:
(a) All succeeding members differ by -CH, unit.
(b) Melting point and boiling point increases with increasing molecular mass.
(c) The difference in molecular masses between two successive members is 16 u.
(d) C₂H₂ and C, H, are NOT the successive members of the alkyne series.
The correct statements are –
(A) (a) and (b)
(B) (b) and (c)
(C) (a) and (c)
(D) (c) and (d)
7. Identify the correct statement about the following reaction:
2H₂S + SO₂ → 2H2O+S
(A) H₂S is oxidising agent and SO, is reducing agent.
(B) H₂S is reduced to sulphur.
(C) SO₂ is oxidising agent and H.S is reducing agent.
(D) SO₂ is oxidised to sulphur.
8. In the given diagram the leaf shown belongs to which plant?
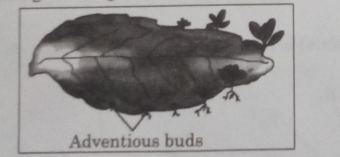
(A) Hibiscus
(B) Money plant
(C) Mustard
(D) Bryophyllum
9. Select out of the following a gland which does NOT occur as a pair in the human body:
(A) Pituitary
(B) Ovary
(C) Testis
(D) Adrenal
10. In human respiratory system, when a person breathes in, the position of ribs and diaphragm will be:
(A) lifted ribs and curve/dome shaped diaphragm.
(B) lifted ribs and flattened diaphragm.
(C) relaxed ribs and flattened diaphragm.
(D) relaxed ribs and curve/dome shaped diaphragm.
11. Which of the following statement(s) is (are) true about human heart?
(a) Right atrium receives oxygenated blood from lungs through pulmonary artery.
(b) Left atrium transfers oxygenated blood to left ventricle which sends it to various parts of the body.
(c) Right atrium receives deoxygenated blood through vena cava from upper and lower body.
(d) Left atrium transfers oxygenated blood to aorta which sends it to different parts of the body.
(A) (a)
(B) (a) and (d)
(C) (b) and (c)
(D) (b) and (d)
12. A cross made between two pea plants produces 50% tall and 50% short pea plants. The gene combination of the parental pea plants must be
(A) Tt and Tt
(B) TT and Tt
(C) Tt and tt
(D) TT and tt
13. Study the I-V graph for three resistors of resistances R₁, R2 and R3 and select the correct statement from the following:
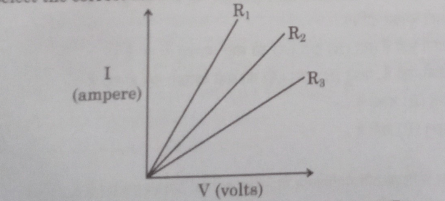
(A) R₁=R2=R3
(B) R₁>R2>R3
(C) R3>R2>R₁
(D) R2>R3>R₁
14. The maximum resistance of a network of five identical resistors of 1/5 Ω each can be –
(A) 1Ω
(B) 0.5 Ω
(C) 0.25 Ω
(D) 0.1 Ω
15. The speed of light in a vacuum is 3 ×108 m/s. If the speed of light in a medium is 2.25 x 108 m/s, the absolute refractive index of the medium is
(A) 7/6
(B) 5/4
(C) 4/3
(D) 3/2
16. Study the following statements:
(a) A fuse in a circuit prevents damage to the circuit due to overloading.
(b) Total resistance in a circuit increases due to overloading.
(c) During short circuiting the current in the circuit abruptly increases.
(d) In order that each appliance has same current, they are connected in parallel to each other. The correct statements are
(A) (a) and (b)
(C) (a) and (c)
(B) (b) and (d)
(D) (a), (c) and (d)
Q. No. 17 to 20 are Assertion – Reason based questions:
These questions consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(A) Both (A) and (R) are true and (R) is the correct explanation of (A).
(B) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
17. Assertion (A): Carbon reduces the oxides of Sodium and Magnesium.
Reason (R): Sodium and Magnesium have more affinity for Oxygen than Carbon.
18. Assertion (A): The deflection of a compass needle placed near a current carrying wire decreases when the magnitude of an electric current in the wire is increased.
Reason (R): Strength of the magnetic field at a point due to a current carrying conductor increases on increasing the current in the conductor.
19. Assertion (A): The colour of clear sky appears blue.
Reason (R): Light of blue colour has longer wavelength as compared to the light of red colour so it is scattered more in the upper atmosphere.
20. Assertion (A): Human female has a perfect pair of sex chromosome.
Reason (R): Sex chromosome contributed by the human male in the zygote decides the sex of a child.
Class 10 Science Answer Key 2024 SET 31/5/3
1. Select from the following the conditions responsible for the rapid spread of bread mould on a slice of bread:
(i) Formation of large number of spores
(ii) Presence of moisture and nutrients in bread
(iii) Low Temperature
(iv) Presence of Hyphae
(A) (i) and (ii)
(B) (ii) and (iv)
(C) (ii) and (iii)
(D) (iii) and (iv)
Answer: (A) (i) and (ii)
2. The incorrect statement about placenta is :
(A) It is a disc embedded in the uterine wall.
(B) It contains villi on the embryo’s side of the tissue.
(C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
(D) The embryo gets nutrition from the mother’s blood through it.
Answer: (C) It has a very small surface area for glucose and oxygen to from mother to the embryo.
3. An aqueous solution ‘A’ turns phenolphthalein solution pink. When another aqueous solution ‘B’ is added to the pink solution, the pink colour disappears. Now when a few drops of solution ‘A’ are added to this reaction, the mixture appears pink again. The respective changes in the nature of the solution are from :
(A) acidic → basic → basic
(B) basic → acidic → acidic
(C) acidic → basic → acidic
(D) basic → acidic → basic
Answer: (D) basic → acidic → basic
4. The correct sequence of events when someone’s hand touches a hot object unconsciously :
(A) Receptors in skin → Motor neuron → Relay neuron → Sensory neuron → Effector muscle in arm
(B) Receptors in skin → Relay neuron → Sensory neuron → Motor neuron → Effector muscle in arm
(C) Receptors in skin → Sensory neuron → Relay neuron → Motor neuron → Effector muscle in arm
(D) Receptors in skin → Sensory neuron → Effector muscle in arm → Motor neuron → Relay neuron
Answer: (C) Receptors in skin → Sensory neuron → Relay neuron → Motor neuron Effector muscle in arm
5. To balance the following chemical equation, the values of the coefficients x, y and & must be respectively :
Zn(NO3)2 A → y ZnO + z NO2 + O2
(A) 4, 2, 2
(B) 2, 2, 4
(C) 4, 4, 2
(D) 2, 4, 2
Answer: (B) 2, 2, 4
6. Which of the following is a redox reaction, but not a combination reaction?
(A) C+ O2→CO2
(B) 2 Mg + O2 → 2 MgO
(C) 2H2 + O2→2 H2O
(D) Fe2O3+ 3CO → 2 Fe + 3CO2
Answer: (D) Fe2O3+ 3CO → 2 Fe + 3CO2
7. An aqueous solution of sodium chloride is prepared in distilled water. The pH of this solution is:
(A) 6
(B) 8
(С) 7
(D) 3
Answer: (C) 7
8. A metal X’ is used in thermit process. When X’ is heated with oxygen, it gives an oxide Y, which is amphoteric in nature. X’ and Y respectively are :
(A) Mn, MnO2
(C) Fe, Fe2O3
(B) Al, Al2O3
(D) Mg, MgO
Answer: (B) Al, Al2O3
9. The process in which transport of soluble products of photosynthesis takes place in plants is known as :
(A)Transpiration
(C) Conduction
(B) Evaporation
(D) Translocation
Answer: (D) Translocation
10. Sense organ in which olfactory receptors are present is
(A) Nose
(B) Skin
(C)Tongue
(D)Inner ear
Answer: (A) Nose



Answer: (C) Reflection, Dispersion and Internal Reflection
15. The colour of light for which the refractive index of glass is minimum, is:
(A) Red
(B) Yellow
(C)Green
(D) Violet
Answer: (A) Red
16. The current carrying device which produces a magnetic field similar to that of a bar magnet is :
(A) A straight conductor
(B) A circular loop
(C) A solenoid
(D) A circular coil
Answer: (C) A Solenoid
For Questions number 17 to 20, two statements are given — one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A) : Electrons move from lower potential to higher potential in a conductor.
Reason (R) : A dry cell maintains electric potential difference across the ends of a conductor.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
18. Assertion (A) : Some vegetable oils are healthy.
Reason (R): Vegetable oils generally have long unsaturated carbon chains.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
19. Assertion (A): Sex of the children will be determined by what they inherit from their mother.
Reason (R): Women have XX sex chromosomes.
Answer: (D) Assertion (A) is false, but Reason (R) is true.
20. Assertion (A): Green plants trap only 1% of the energy of sunlight that falls on their leaves.
Reason (R) : All green plants are the producers in a food chain.
Questions no. 21 to 26 are very short answer type questions.
21. (a) Sometimes while running, the athletes suffer from muscle cramps. Why? How is the respiration in this case different from aerobic respiration?
OR (b) Write the other name given to lymph. State its two functions.
22. Write the formula and the molecular mass of the third homologue of alcohols. State how the boiling point of an alcohol changes as one moves from lower to higher homologues.
23. (a) Copper powder is taken in a china dish and heated over a burner. Name the product formed and state its colour. Write the chemical equation for the reaction involved.
OR (b) Write chemical equation for the chemical reaction which occurs when the aqueous solutions of barium chloride and sodium sulphate react together. Write the symbols of the ions present in the compound precipitated in the reaction.
24. Identify the organ in the human female reproductive system where the sperm encounters the egg cell. What will happen if it is blocked ? Name the technique by which it can be blocked.
25. The linear magnification produced by a spherical mirror is +3.” Based on this statement answer the following questions :
(a) What is the type of mirror?
(b) Where is the object located ?
(c) List two properties of the image formed (other than the size/magnification).
26. The filament of an electric lamp draws a current of 0•5 A, which lights for 2 hours. Calculate the charge that flows through the circuit.
27. Answer the following questions in the context of electrolysis of water :
(a) Why is this reaction/process called a decomposition reaction?
(b) Giving reason state whether this reaction is exothermic or endothermic.
(c) Name the gases collected at the anode and cathode.
(d) What is the mass ratio of the gases collected at the anode and cathode ?
28. Differentiate between food chain and food web. In a food chain consisting of deer, grass and tiger, if the population of deer decreases, what will happen to the population of organisms belonging to the first and third trophic levels?
29. Name a plant growth hormone synthesized at the shoot tip. Explain its effect on the growth of a plant in response to light.
30. Name the ore of mercury and state the form in which it is found in nature. Write the chemical equations along with the condition required for the reactions involved in the extraction of mercury from its ore.
31. Mendel crossed pure tall pea plants (TT) with pure short pea plants (tt) and obtained F, progeny. When the plants of F, progeny were self-pollinated, plants of F2 progeny were obtained.
(a) What did the plants of F1 progeny look like? Give their gene combination.
(b) Why could the gene for shortness not be expressed in plants of Fi progeny?
(c) Write the ratio of the plants obtained in F2 progeny and state the conclusion that can be drawn from this experiment.
Class 10 Science Answer Key 2024 SET 3 (31/1/3)
1. When 2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as:
(a) NaOH + Zn → NaZnO2 + H20
(b) 2NaOH + Zn → Na2ZnO2 + H2
(c) 2NaOH + Zn → NaZnO2 + H2
(d) 2NaOH + Zn → Na2ZnO2 + H2O
Answer: (b) 2NaOH + Zn → Na2ZnO2 + H2
2. Select from the following a decomposition reaction in which source of energy for decomposition is light:
(a) 2FeSO4 → Fe203+ SO2+ SO3
(b) 2H2O → 2H2+ O2
(c) 2AgBr → 2Ag + Br2
(d) CaCO3 → CaO + CO2
Answer: (c) 2AgBr → 2Ag + Br2
3. A metal and a non-metal that exists in liquid state at the room temperature are respectively :
(a) Bromine and Mercury
(b) Mercury and lodine
(c) Mercury and Bromine
(d) Iodine and Mercury
Answer: (c) Mercury and Bromine
4. Carbon compounds:
(i) are good conductors of electricity.
(ii) are bad conductors of electricity.
(iii) have strong forces of attraction between their molecules.
(iv) have weak forces of attraction between their molecules.
The correct statements are:
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iii)
Answer: (d) (i) and (iii)
5. Consider the following compounds :
FeSO4; CuSO4; CaSO4; Na2CO3
The compound having maximum number of water of crystallisation in its crystalline form in one molecule is :
(a) FeSO4
(b) CuSO4
(c) CaSO4
(d) Na2CO3
Answer: (d) Na2CO3 ; 10 water molecules
6. Oxides of aluminium and zine are :
(a) acidic
(b) basic
(c) amphoteric
(d) neutral
Answer: (c) amphoteric; behave as both acidic and basic oxides
7. MnO2 + 4HCI → MnCl2 + 2H2O + Cl2
The reaction given above is a redox reaction because in this case
(a) MnO2 is oxidised and HCI is reduced.
(b) HCI is oxidised.
(c) MnO2 is reduced.
(d) MnO2 is reduced and HCI is oxidised.
Answer: (d) MnO2 is reduced and HCI is oxidised; MnO2 loses its oxygen to form MnCl2 and HCl loses its H to form Cl2.
8. Consider the following statements
(i) The sex of a child is determined by what it inherits from the mother.
(ii) The sex of a child is determined by what it inherits from the father.
(iii) The probability of having a male child is more than that of a female child.
(iv) The sex of a child is determined at the time of fertilisation when male and female gametes fuse to form a zygote.
The correct statements are :
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) i), (iii) and (iv)
Answer: (b) (ii) and (iv)
9. Chromosomes :
(i) carry hereditary information from parents to the next generation.
(ii) are thread like structures located inside the nucleus of an animal cell.
(iii) always exist in pairs in human reproductive cells.
(iv) are involved in the process of cell division.
The correct statements are :
(a) (i) and (ii)
(b) (iii) and (iv)
(a) (i), (ii) and (iv)
(d) (i) and (iv)
Answer: (a) (i) and (ii)
10. In a nerve cell, the site where the electrical impulse is converted into a chemical signal is known as :
(a) Axon
(b) Dendrites
(c) Neuromuscular junction
(d) Cell body
Answer: (c) Neuromuscular junction; a neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
11. A stomata closes when:
(i) it needs carbon dioxide for photosynthesis.
(ii) it does not need carbon dioxide for photosynthesis.
(iii) water flows out of the guard cells.
(iv) water flows into the guard cells.
The correct reason(s) in this process is/are :
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: (c) (ii) and (iii);
12. At what distance from a convex lens should an object be placed to get an image of the same size as that of the object on a screen?
(a) Beyond twice the focal length of the lens.
(b) At the principal focus of the lens.
(c) At twice the focal length of the lens.
(d) Between the optical centre of the lens and its principal focus.
Answer: (c) At twice the focal length of the lens;
13. The lens system of human eye forms an image on a light sensitive screen, which is called as:
(a) Cornea
(b) Ciliary muscles
(c) Optic nerves
(d) Retina
Answer: (d) Retina
Class 10 Science Question Paper 2024 PDF Download
On March 02, 2024, the Central Board of Secondary Education successfully conducted the Class 10th Science Exam 2024. The CBSE Class 10th Science Question Paper was for a total of 100 marks out of which 80 marks were for the theory exam and the rest 20 marks were for the internal assessments. The CBSE Board Question Papers are valuable resources as after the successful conduction of the exam, these question papers help the students to evaluate their performance in the exams. They also give an overview of papers to students preparing to appear in the class 10th exams next year of the types. To download the CBSE Class 10 Science Question Paper 2024 set 1, 2, and 3 PDF, the direct link has been updated below:
| CBSE Class 10 Science Question Paper 2024 PDF | |
| Class 10 Science Exam Paper 2024 SET | Question Paper Link |
| SET 1 | CBSE Class 10th Science Set 1 Question Paper 2024 |
| SET 2 | CBSE Class 10th Science Set 2 Question Paper 2024 |
| SET 3 | CBSE Class 10th Science Set 3 Question Paper 2024 |
| SET 31/4/3 | CBSE Class 10 Science Question Paper 2024 SET 31-4-3 |
| SET 31/1/3 | CBSE Class 10 Science Question Paper 2024 SET 31-1-3 |
| SET 31/2/3 | CBSE Class 10 Science Question Paper 2024 SET 31-2-3 |
| SET 31/4/2 | CBSE Class 10 Science Question Paper 2024 SET 31-4-2 |
Class 10 Science Answer Key 2024 PDF Download
The Central Board of Secondary Education successfully conducted the CBSE Board Class 10th Science Examination today i.e., March 02, 2024. Students who appeared in the examination can able to check the complete CBSE Class 10 Science Answer Key PDF 2024 for all the sets from the direct link that has been shared in the article below. To help the students, a CBSE Class 10 Science Answer Key 2024 designed by our expert faculty has been shared with students in the article here.
CBSE Class 10 Science Exam Analysis 2024
All the students who appeared in the Class 10 Science Exam 2024 for SET 1, 2, 3, can check the live Class 10 Science Exam Analysis 2024 by Sankalp Bharat Foundation. Watch the video.
CBSE Class 10 Science Exam Paper Analysis 2024
Most of the students who appeared for the CBSE Class 10 Science Exam today i.e., March 02, 2024 found the paper a bit easy and well-structured. Students have mentioned that all questions were based on the syllabus and followed the same format as the latest CBSE class 10th Science sample paper 2024. Overall it was a standard paper, based on the NCERT syllabus and easy for the students who have practised through the sample papers. The CBSE class 10 Science exam analysis shared here is entirely based on the review of the paper shared by students and expert educators.
Vigyata said she was nervous before the science paper but now she is very happy as the paper went very well.
Ansh also reiterated that it was not a lengthy paper except for some questions that needed more Comprehension.
Some of the general views of the students have been shared below:
- The overall Difficulty Level of the CBSE Class 10th Science Question paper 2024 was Moderate to Easy.
- All questions were based on the latest syllabus and followed the pattern of the CBSE sample paper.
- MCQs were mostly average and needed conceptual knowledge.
- Case-based questions were easy to moderate.
- No question was found to be outside of the syllabus.
- About 60-70% of the questions were straight from the textbook.
- When students were asked whether the Paper was Lengthy? They replied that Yes, the paper was lengthy but manageable.



 CBSE Class 10th Maths Answer Key 2026, C...
CBSE Class 10th Maths Answer Key 2026, C...
 CUET Legal Study Previous Year Question ...
CUET Legal Study Previous Year Question ...
 NIMCET Final Answer Key 2025 Out, Downlo...
NIMCET Final Answer Key 2025 Out, Downlo...













