Define Organic Compounds
Organic compounds are molecules that contain carbon atoms bonded to hydrogen, along with other elements like oxygen, nitrogen, sulfur, and more. These compounds form the basis of life and are crucial to living organisms. Carbon’s unique ability to form stable bonds with itself and other elements allows for the vast diversity of organic molecules. Organic compounds can be simple, like methane (CH4), found in natural gas, or complex, such as proteins, carbohydrates, and DNA. These compounds play fundamental roles in biological processes.
For instance, proteins are essential for cell structure and functions, while carbohydrates provide energy. DNA carries genetic information, guiding the development and functioning of living organisms. Organic chemistry explores the structures, properties, and reactions of these compounds. Despite the complexity of life’s organic molecules, their understanding is vital for comprehending the intricacies of Biology, medicine, and various industrial applications.
Types of Organic Compounds
Organic Compounds are diverse and can be categorized into several types namely: Alkanes, alkenes, alkynes, aromatic compounds, Alcohols, Aldehydes, Ketones, and many more. Here we have discussed some of them. These are just a few examples, and organic chemistry encompasses a vast array of compounds with different structures and functions.
| Different Types of Organic Compounds | |
| Types Of Compounds | Description |
| Alkane | Saturated hydrocarbons with single bonds (e.g., methane, ethane). |
| Alkene | Hydrocarbons with at least one carbon-carbon double bond (e.g., ethene, propene). |
| Alkynes | Hydrocarbons with at least one carbon-carbon triple bond (e.g., ethyne, propyne). |
| Aromatic Compounds | Ring-shaped structures with alternating double bonds, like benzene. |
| Alcohols | Compounds with hydroxyl (-OH) functional groups attached to carbon atoms (e.g., ethanol). |
| Aldehydes | Compounds with a carbonyl group (C=O) at the end of the carbon chain (e.g., formaldehyde). |
| Ketones | Compounds with a carbonyl group (C=O) within the carbon chain (e.g., acetone). |
| Carboxylic Acid | Compounds with a carboxyl group (COOH) at the end of the carbon chain (e.g., acetic acid). |
| Esters | Derived from carboxylic acids, with an -O-R group replacing the -OH of the carboxyl group. |
| Amines | Compounds containing a nitrogen atom bonded to hydrogen or carbon atoms (e.g., ammonia, amines). |
| Amides | Derived from carboxylic acid with a nitrogen atom bonded to the carbonyl carbon. |
| Ethers | Compounds with an oxygen atom linking two hydrocarbon groups. |
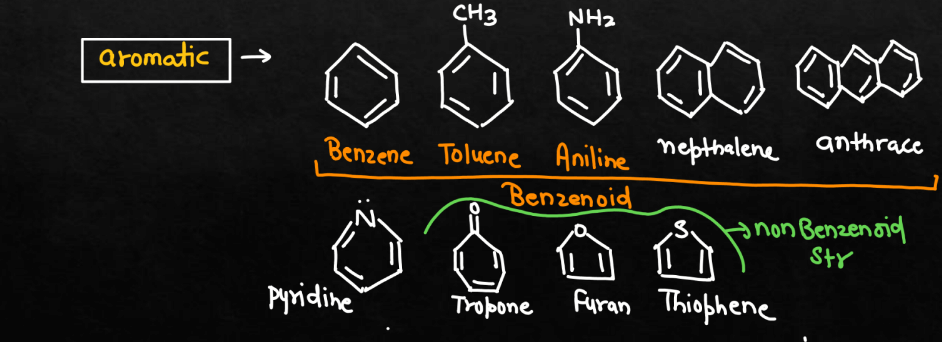
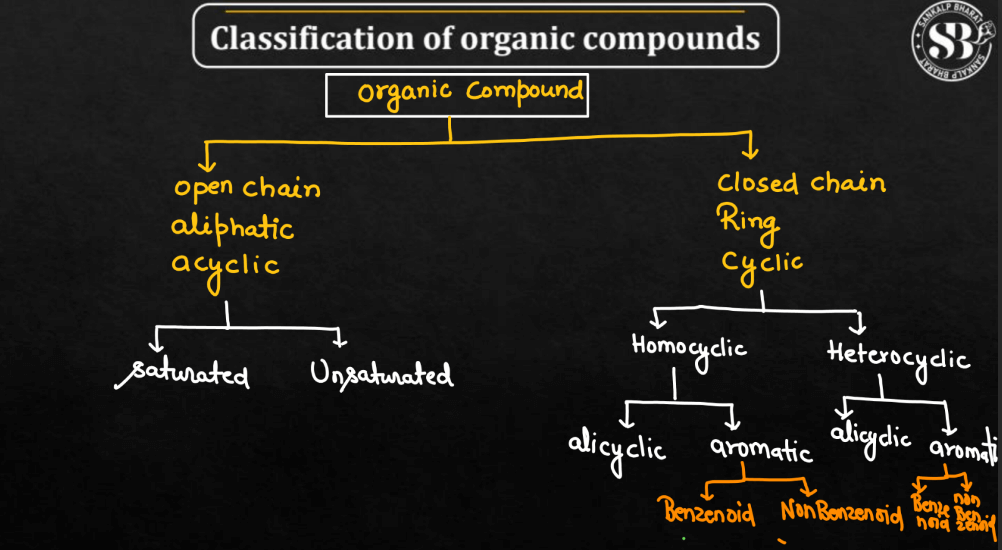
Classification of Organic Compounds
Organic Compounds can be classified based on their structure, functional groups, sources, and isomerism. Here we have discussed some of the common classifications of organic compounds. These classifications help organize the diverse world of organic compounds and provide a framework for understanding their properties and reactivities.
1. Based on Structure
Below we have described some compounds that will help you understand the organic compounds based on their structures.
| Compounds | Description |
| Open-Chain compounds | These compounds have a straight or branched chain of carbon atoms |
| Closed-Chain Compounds | These compounds have a ring or cyclic structure. |
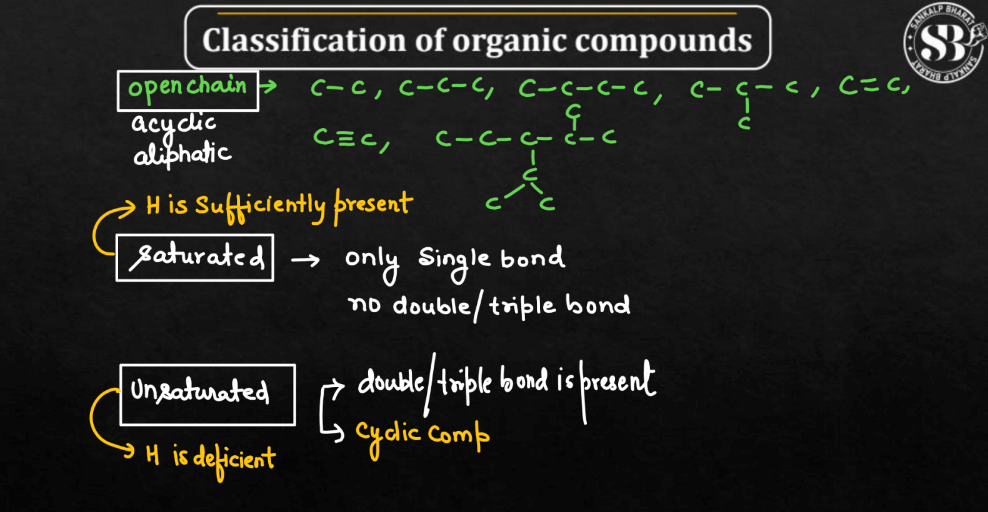
Open-Chain Compounds
The Open-chain compounds are like molecular trains – they have a straight or branched structure with carbon atoms forming a continuous chain. Each “carriage” represents a carbon chain, and these compounds lack a closed ring. Examples of open-chain compounds include alkanes, alkenes, and alkynes. Their straightforward structure contracts with the closed loops of cyclic or ring-shaped compounds.
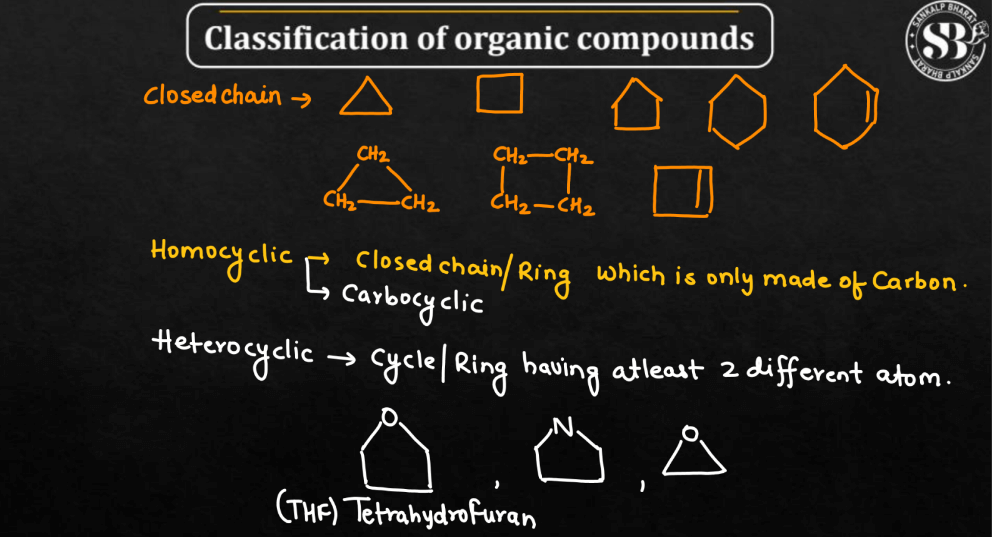
Closed-Chain Compounds
The closed-chain compounds, unlike molecular trains, are like circular bracelets. Carbon atoms form a closed ring, creating a cyclic structure. This contrasts with open-chain compounds, as there’s no clear beginning or end. Benzene is an example, featuring a stable ring of carbon atoms with alternating single and double bonds.
2. Based on Functional Groups:
Below we have described some compounds that will help you understand the organic compounds based on their Functional Groups.
- Hydrocarbons: The hydrocarbons contain only carbon and hydrogen. For example Alkanes, Alkenes, and Alkynes.
- Alcohols: Alcohols contain the -OH Hydroxyl group at the end of the carbon chain.
- Aldehydes: Aldehydes contain the C=O Carbonyl group at the end of the carbon chain.
- Ketone: Ketones contain the C=O group within the carbon chain.
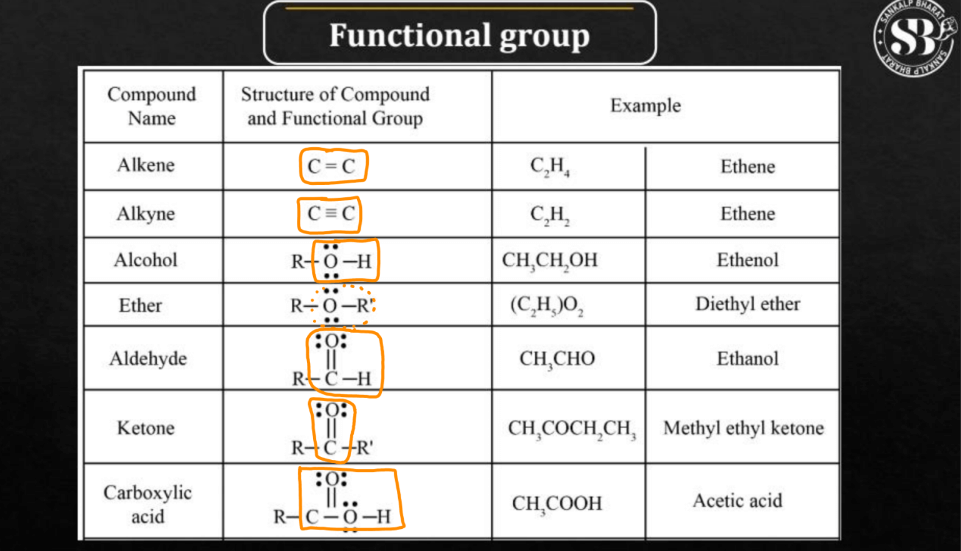
- Carboxyl Group: They contain the COOH carboxyl group.
- Esters: They are derived from carboxylic acids, with an -O-R group.
- Amines: Amines contain a nitrogen atom bonded to hydrogen or carbon atoms.
- Amides: Amides are derived from carboxylic acids, with a nitrogen atom bonded to the carbonyl carbon.
- Ethers: They contain an oxygen atom linking two hydrocarbon groups.
3. Based on Sources
Below we have described some compounds that will help you understand the organic compounds based on their Sources.
| Compounds | Description |
| Natural Compounds | The natural compounds are derived from living organisms (e.g., sugars, amino acids, lipids). |
| Synthetic Compounds | Synthetic compounds are created through chemical synthesis (e.g., pharmaceuticals, plastics). |
Natural Compounds
Natural compounds are like nature’s building blocks – they originate from living organisms. Found in plants, animals, and microorganisms, they include sugar, amino acids, and lipids. These compounds play a vital roles in biological processes, making them essential for life. Examples are glucose in fruits and proteins in muscles.
Synthetic Compounds
Synthetic compounds are human-made marvels, crafted through chemical processes rather than occurring naturally. Think of them as tailor-made creations – pharmaceuticals, plastics, and artificial materials fall into this category. Scientists and engineers design and produce these compounds to serve specific purposes, from medicine to everyday products like clothing and packaging.
4. Based on Isomerism
Below we have described some compounds that will help you understand the organic compounds based on Isomerism.
| Compounds | Description |
| Structural Isomers | Structural isomers have the same molecular formula but different structural arrangements. |
| Stereoisomers | Stereoisomers have the same structural formula but differ in spatial arrangement (e.g., cis-trans isomers, enantiomers). |
Structural Isomers
Structural isomers are molecules with the same molecular formula but different arrangements of atoms. Think of them like rearranged LEGO sets – same pieces, different structures. This structural variability results in distinct chemical properties, influencing how they interact in biological and chemical processes.
Stereoisomers
Stereoisomers are like molecular twins with the same ingredients but a different arrangement. They have identical chemical formulas but differ in how the atoms are arranged in space. Think of them as a mirror image or molecules that, despite having the same parts, cannot be superimposed on each other, like left and right hands.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













