In chemistry, various tests are used to identify different substances. Benedict’s test detects reducing sugars. The iodine test checks for starch presence, turning from brown to blue-black. Biuret test identifies proteins, turning violet from blue. Litmus paper tests acidity or alkalinity, changing color accordingly. pH paper measures the pH levels of a solution. These tests help scientists analyze diverse compounds and reactants. Here we have briefly discussed Benedict’s test below in the article.
What is Benedict’s Test?
Benedict’s test is a chemical test used to detect the presence of reducing sugars, such as glucose, in a solution. It involves heating the sample with Benedict’s reagent (a blue solution containing copper sulfate and sodium citrate) in a boiling water bath. If reducing sugars are present, the blue solution changes color to green, yellow, orange, or red, depending on the amount of reducing sugar present. This color change indicates a positive result for the presence of reducing sugars. Benedict’s test is commonly used in biochemical and food science laboratories to analyze sugar content in various samples.
In simple terms, Benedict’s test is like a special experiment to find out if there’s sugar in a liquid. You mix the liquid with a blue solution, and if the liquid has sugar, it changes color. This helps scientists and cooks know if there’s sugar in what they are testing.
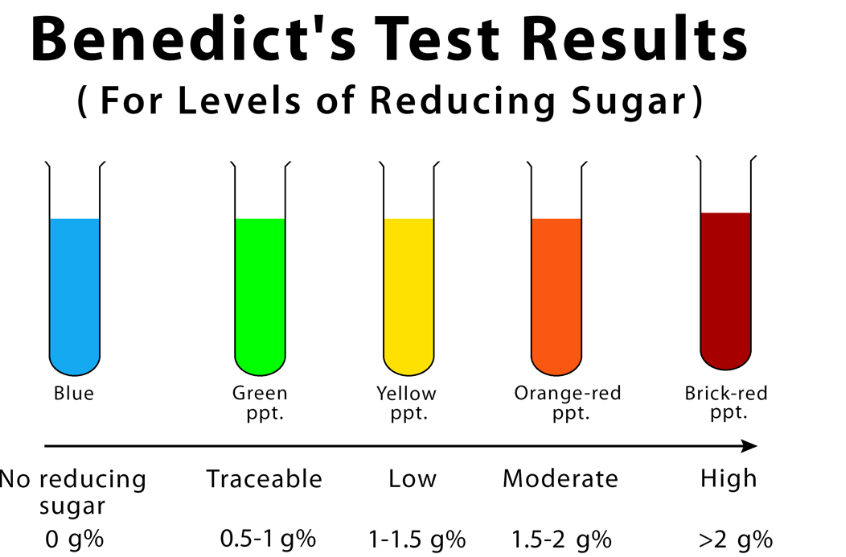
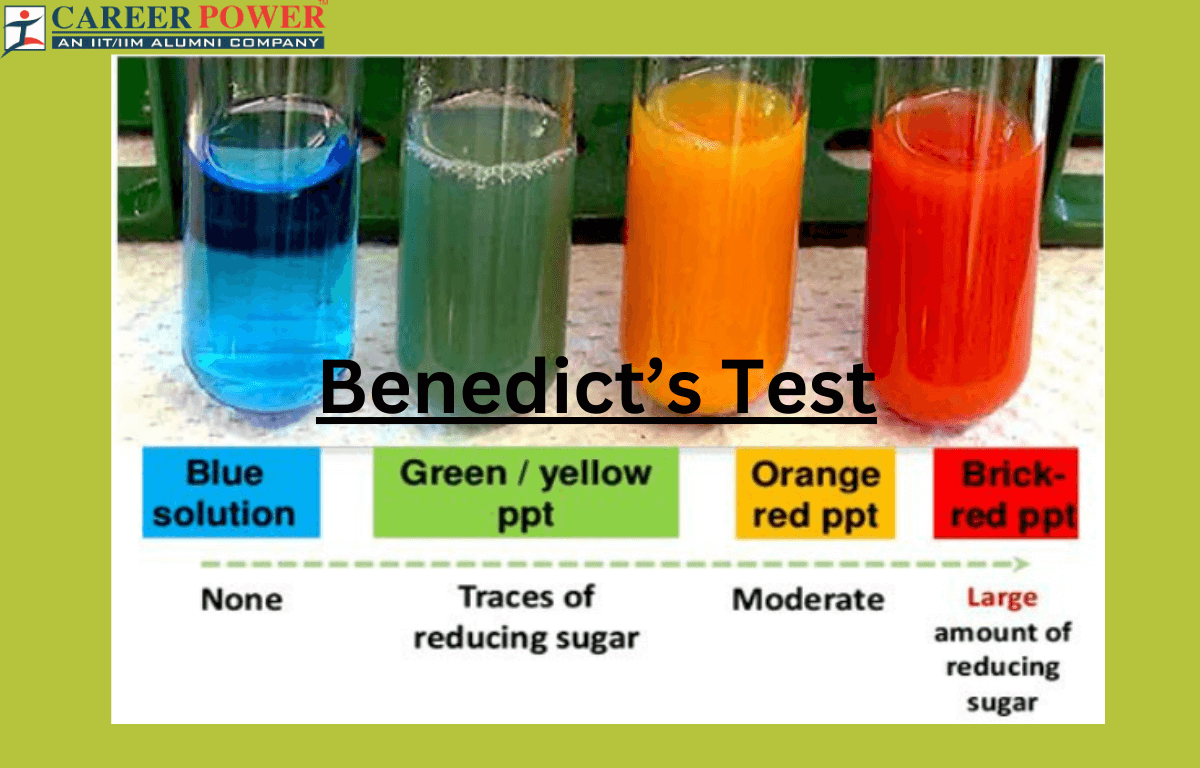
Benedict’s test results in a color change ranging from blue (negative, no reducing sugars) to green, yellow, orange, or red (positive, presence of reducing sugars). The intensity of the color change indicates the amount of reducing sugars present.
Benedict’s Reagent and its Composition
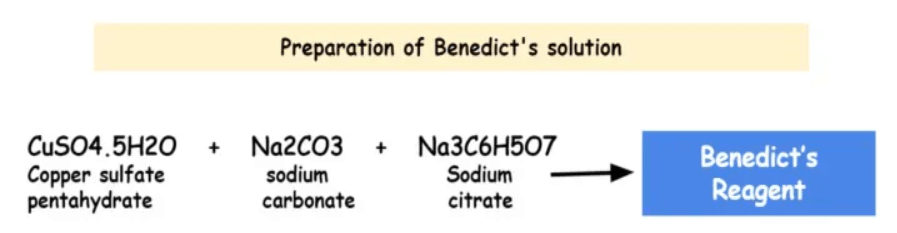
Benedict’s reagent, also known as Benedict’s solution, is a chemical reagent used to test for the presence of reducing sugars. The exact proportions of these components can vary slightly depending on the specific formulation of Benedict’s reagent used.
When a reducing sugar is present and heated with Benedict’s reagent, a redox reaction occurs, resulting in the reduction of copper (II) ions to copper (I) ions. This reduction is indicated by a color change in the solution, usually to green, yellow, orange, or a brick-red precipitate, depending on the concentration of reducing sugars. The composition of Benedict’s reagent typically includes:
| Composition of Benedict’s Reagent | ||
| Components | Chemical Formula | Function |
| Copper (II) Sulfate | CuSO4 | Provides copper ions for the reaction with reducing sugars. Gives the reagent its blue color. |
| Sodium Carbonate | Na2CO3 | Acts as a basic stabilizer, maintaining the alkaline pH of the solution and preventing the precipitation of copper (II) hydroxide. |
| Sodium Citrate | C6H5Na3O7 | Acts as a complexing agent, preventing the precipitation of copper (II) ions as copper (II) hydroxide. Helps in maintaining the stability of the reagent. |
Principle of Benedict’s Test
Benedict’s Test works on the principle that certain substances, like reducing sugars (eg., glucose and fructose), have the ability to reduce copper (II) ions to copper (I) ions when heated in an alkaline solution. Benedict’s reagent contains copper sulfate, which is blue, and when it reacts with reducing sugars, it forms a reddish-brown copper (I) oxide precipitate. The color changes from blue to green, yellow, orange, or red indicate the quantity of reducing sugars in the solution being tested.
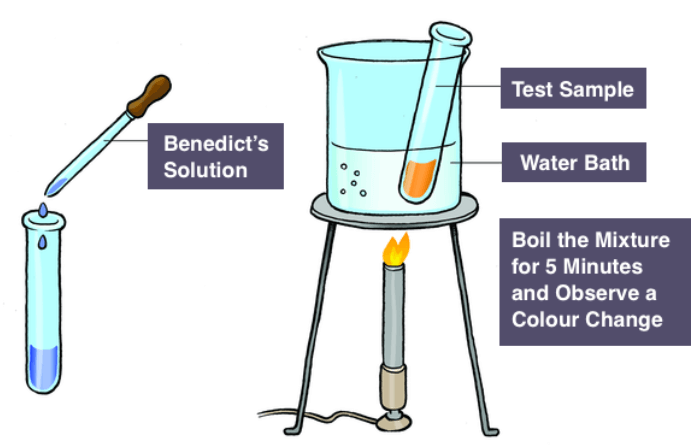
Procedure of Benedict’s Test
Benedict’s test is a chemical test used to detect the presence of reducing sugars, such as glucose and maltose, in a given solution. Remember that Benedict’s test is specific for reducing sugars and may not give accurate results for all types of carbohydrates. Also, it’s essential to handle the chemicals safely and dispose of them properly after the experiment. Here’s the procedure for Benedict’s test:
Material Needed
- Benedict’s reagent (copper sulfate solution mixed with sodium citrate and sodium carbonate).
- Water bath or a heat source.
- Test tubes.
- Pipette.
- Stirring rod.
- Water.
- Sugar solution to be tested.
Procedure
- Prepare the Sample: Start by preparing a solution containing the sugar you want to test. Dissolve a small amount of sugar in water to create a clear solution.
- Prepare Benedict’s Reagent: Take Benedict’s reagent in a test tube. It usually comes in a blue color.
- Mix the Sample with Benedict’s Reagent: Using a pipette, add a small amount of your prepared sugar solution to the test tube containing Benedict’s reagent.
- Heat the Mixture: Place the test tube in a boiling water bath or heat it over a flame. Heat the mixture gently, avoiding vigorous boiling, for a few minutes (usually 3-5 minutes).
- Observe the Color Change: If reducing sugars are present in the solution, the blue color of Benedict’s reagent will change to green, yellow, orange, or even a brick-red precipitate, depending on the concentration of reducing sugars in the sample. The color change indicates the presence of reducing sugars.
- Interpret the Result: The intensity of the color change can give you an idea about the amount of reducing sugars present. A higher concentration of reducing sugars will result in a more intense color change.
Uses of Benedict’s Test
Benedict’s Test is a chemical test used primarily for detecting the presence of reducing sugars in a given solution. It’s important to note that while Benedict’s test is useful for detecting reducing sugars, it is not specific to any particular type of sugar. Differentiating between specific sugars requires additional tests or techniques. Here are some specific uses of Benedict’s test:
- Detecting Reducing Sugars: Benedict’s test is commonly used in Biology and Chemistry laboratories to identify the presence of reducing sugars such as glucose, fructose, and maltose. These sugars can reduce copper (II) ions in the reagent to copper (I) ions, causing a color change in the solution.
- Qualitative Analysis: It’s used qualitatively to distinguish between reducing and non-reducing sugars. Non-reducing sugars, like sucrose, do not react with Benedict’s reagent in their original form.
- Food Science: Benedict’s test is employed in the food industry to detect the presence of reducing sugars in food products. For example, it can be used to test for the presence of sugars in honey or to monitor sugar content in beverages.
- Medical Diagnostics: In some medical tests, Benedict’s test can be used to detect the presence of reducing sugars in urine. Which can indicate certain health conditions, such as diabetes.
- Educational Purposes: Benedict’s test is widely used in educational settings to teach students about chemical reactions, especially redox reactions involving reducing sugars and copper ions.


 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...













